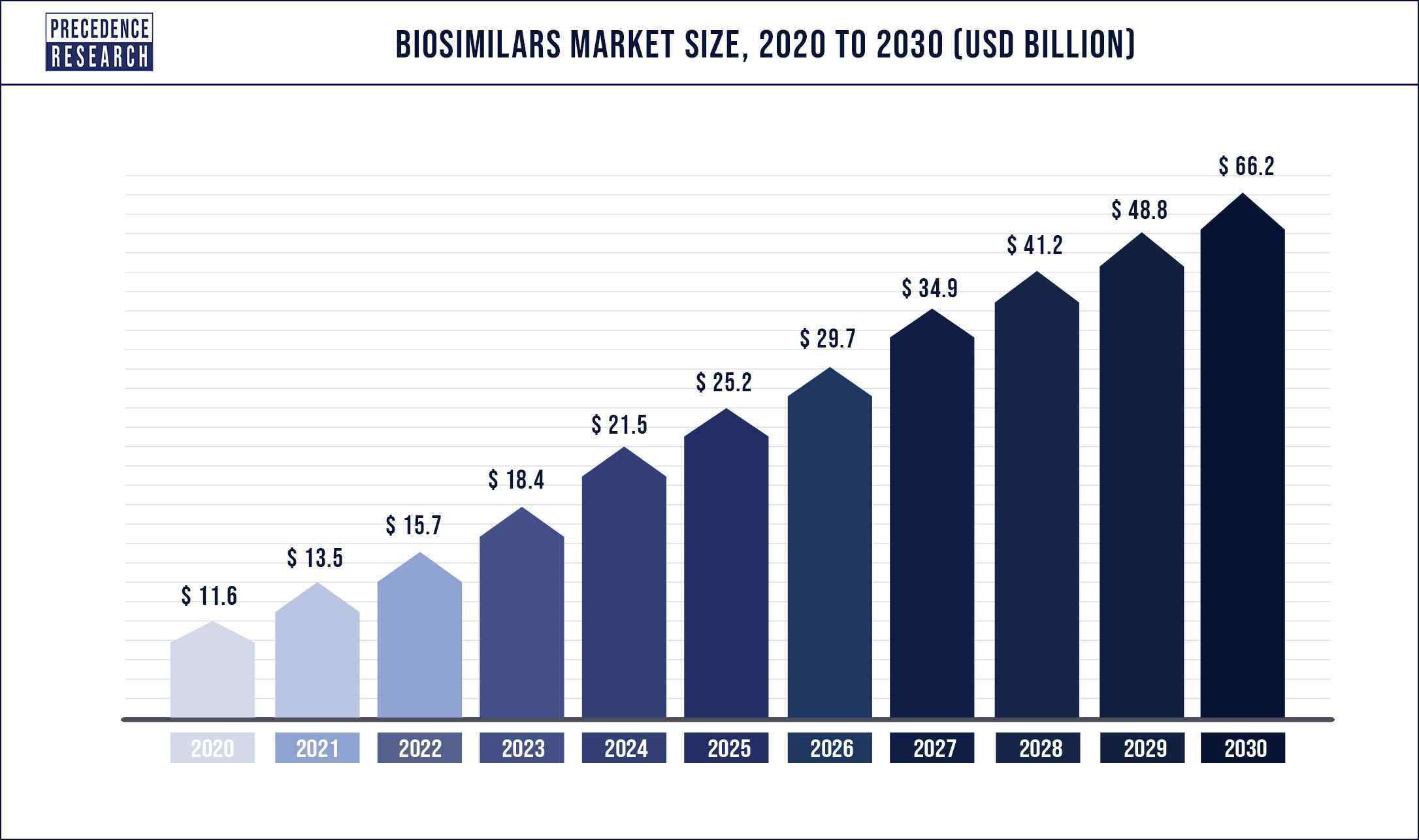
Biosimilars Market Revenue to Reach US$ 66.2 Billion by 2030
According to the industry experts, The biosimilars market garnered $ 13.5 billion in 2021 and is expected to generate $ 66.2 billion by 2030, manifesting a CAGR of 17.5% from 2021 to 2030. The report contains 150+ pages with detailed analysis.
The base year for the study has been considered 2021, the historic year 2019 and 2020, the forecast period considered is from 2021 to 2030. The biosimilars market is analyzed on the basis of value (US$ Million), volume (Unit), and price (US$/Unit).
Download the Sample Pages of this Report for Better Understanding (Including TOC, List of Tables & Figures, and Chart) @ https://www.precedenceresearch.com/sample/1359
The rising prevalence of diseases and the effective treatment of these chronic diseases at affordable costs are the two major drivers of the global biosimilars market. The biosimilar drugs are very efficient at targeting specific infected cells without damaging the normal cells, which is significantly boosting the adoption of the biosimilar drugs among the global population. The rising government initiatives to drive the adoption of various biosimilar drugs by incorporating favorable changes to the regulations is a positive sign for the manufacturers of the biosimilar drugs.
For instance, Europe approved more than 60 biosimilar brands. Europe is a mature market for the biosimilar drugs. The rising adoption and popularity of the biosimilars in the North America and Asia Pacific is estimated to provide lucrative growth opportunities for the market players to expand in the upcoming future. Further, the rising investments by the market players in the research and development activities are expected to provide new growth avenues in the forthcoming years.
Biosimilars Market Scope
This market report studies market dynamics, status and outlook especially in North America, Europe and Asia-Pacific, Latin America, the Middle East and Africa. This research report offers scenario and forecast (revenue/volume), and categorizes the market by key players and various segment. This report also studies global market prominence, competitive landscape, market share, growth rates market dynamics such as drivers, restraints and opportunities, and distributors and sales channels.
This research study also integrates Industry Chain analysis and Porter’s Five Forces Analysis. Further, this report offers a competitive scenario that comprises collaborations, market concentration rate and expansions, mergers & acquisitions undertaken by companies.
Browse Healthcare Research Reports @ https://www.marketstatsnews.com/healthcare/
Europe was the dominating market that accounted for over 50% of the market share in 2020. EMA played an important role in augmenting the adoption of the biosimilar drugs in the region. Moreover, the increased disposable income, higher adoption rate of advanced technologies, and increased awareness regarding the biosimilars among the population has spurred the growth of the market in Europe. Further, the presence of numerous players in the region and various developmental strategies adopted by them played a crucial role in the market development.
North America region is estimated to be the most opportunistic market during the forecast period. North America is characterized by the increased demand for the advanced and innovative healthcare products, increased consumer expenditure on healthcare, and active participation of the regulatory authorities to overlook the matters related to the preclinical trials and product launches in the market. FDA in US has recently approved around 20 biosimilar products. The rising popularity of the biosimilar drugs among the patients in the US is expected to propel the demand for the biosimilars in North America.
The rising burden of various chronic diseases such as cardiovascular diseases, diabetes, cancer, and kidney failure amongst the global population is boosting the growth of the biosimilars market. The biosimilars provide treatment to various chronic diseases at affordable costs and hence it is gaining popularity among the global consumers.The rising prevalence of diabetes is another major driver that may boost the demand for the biosimilar drugs. According to the World Health Organization, diabetes is the major reason behind the blindness, kidney failure, stroke, and heart attacks. The availability of affordable biosimilar drugs for the treatment of such serious diseases is boosting the market growth.
The complexities related to the manufacturing of the biosimilar drugs and huge capital investments are the major drawbacks that can restrict market growth. Moreover, lack of awareness regarding the availability of biosimilars among the population especially in the developing and underdeveloped regions are the potential factors that may hamper the market growth during the forecast period.
Based on the product, the monoclonal antibodies segment was the dominant segment in 2020. The extensive usage of monoclonal antibodies in the treatment of variousdiseases such as cancer, rheumatoid arthritis, cardiovascular diseases, and multiple sclerosis. This segment accounts for over 25% of the market share. The higher effectiveness of the monoclonal antibodies in the treatment of the cancer and diabetes is expected to drive the segment growth further in the upcoming years.
Based on the application, the oncology segment dominated the market in 2020. According to the International Agency for Research on Cancer, around 19.3 million new cancer cases and around 10 million cancer related deaths were reported in the year 2020, across the globe. Prevalence of breast cancer in the female population is surging, accounting for around 11.7% of the new cancer cases followed by the lungs cancer that accounted for 11.4% and colorectal cancer accounted for 10.0% in 2020. Hence, the growing demand for the biosimilar drugs for the treatment of cancer is boosting the segment growth.
In 2020, Pfizer obtained the FDA approval for its Nyvepria, which is used for lowering the infection incidences.In May 2020, Fresenius Kabi acquired approval for its MSB11455, a pegfilgrastim biosimilar, from both the FDA and EMA. Drug approval is a major strategy adopted by the market players. The various other developmental strategies like acquisition, partnerships, mergers, and government policies fosters market growth and offers lucrative growth opportunities to the market players.
Biosimilars Market Key Players/Manufacturers
This report also provides detailed company profiles of the key market players. This research report also highlights the competitive landscape of the biosimilars market and ranks noticeable companies as per their occurrence in diverse regions across the globe and crucial developments initiated by them in the market space. This research study also tracks and evaluates competitive developments, such as collaborations, partnerships, and agreements, mergers and acquisitions; novel product introductions and developments, promotion strategies and Research and Development (R&D) activities in the marketplace. The competitive profiling of these players includes business and financial overview, gross margin, production, sales, and recent developments which can aid in assessing competition in the market.
Some of the prominent players in the global biosimilars market include:
- Novartis
- Synthon Pharmaceuticals, Inc.
- TevaPharmaceutical Industries Ltd.
- LG Life Sciences
- Celltrion
- Biocon
- Hospira
- Merck Serono
- Biogen idec, Inc.
- Genentech
Biosimilars Market Segments Covered
By Product
- Monoclonal Antibodies
- Glucagon
- Insulin
- Erythropoietin
- Interferon
- Calcitonin
- Others
By Application
- Oncology
- Growth Hormonal Deficiency
- Blood Disorders
- Chronic & Autoimmune Disorders
- Others
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia Pacific
- China
- India
- Japan
- South Korea
- Rest of the World
Biosimilars Market Research Objective
- To provide a comprehensive analysis of the biosimilars industry and its sub-segments in the global market, thereby providing a detailed structure of the industry
- To provide detailed insights into factors driving and restraining the growth of this global market
- To provide a distribution chain analysis/value chain for the this market
- To estimate the market size of the global biosimilars market where 2019 would be the historical period, 2020 shall be the base year, and 2020 to 2027 will be the forecast period for the study
- To provide strategic profiling of key companies (manufacturers and distributors) present across the globe, and comprehensively analyze their competitiveness/competitive landscape in this market
- To analyze the global market in four main geographies, namely, North America, Europe, Asia-Pacific, and the Rest of the World
- To provide country-wise market value analysis for various segments of the biosimilars
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Biosimilars Market
5.1. COVID-19 Landscape: Biosimilars Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Biosimilars Market, By Product
8.1. Biosimilars Market, by Product Type, 2021-2030
8.1.1. Monoclonal Antibodies
8.1.1.1. Market Revenue and Forecast (2019-2030)
8.1.2. Glucagon
8.1.2.1. Market Revenue and Forecast (2019-2030)
8.1.3. Insulin
8.1.3.1. Market Revenue and Forecast (2019-2030)
8.1.4. Erythropoietin
8.1.4.1. Market Revenue and Forecast (2019-2030)
8.1.5. Interferon
8.1.5.1. Market Revenue and Forecast (2019-2030)
8.1.6. Calcitonin
8.1.6.1. Market Revenue and Forecast (2019-2030)
8.1.7. Others
8.1.7.1. Market Revenue and Forecast (2019-2030)
Chapter 9. Global Biosimilars Market, By Application
9.1. Biosimilars Market, by Application, 2021-2030
9.1.1. Oncology
9.1.1.1. Market Revenue and Forecast (2019-2030)
9.1.2. Growth Hormonal Deficiency
9.1.2.1. Market Revenue and Forecast (2019-2030)
9.1.3. Blood Disorders
9.1.3.1. Market Revenue and Forecast (2019-2030)
9.1.4. Chronic & Autoimmune Disorders
9.1.4.1. Market Revenue and Forecast (2019-2030)
9.1.5. Others
9.1.5.1. Market Revenue and Forecast (2019-2030)
Chapter 10. Global Biosimilars Market, Regional Estimates and Trend Forecast
10.1. North America
10.1.1. Market Revenue and Forecast, by Product (2019-2030)
10.1.2. Market Revenue and Forecast, by Application (2019-2030)
10.1.3. U.S.
10.1.3.1. Market Revenue and Forecast, by Product (2019-2030)
10.1.3.2. Market Revenue and Forecast, by Application (2019-2030)
10.1.4. Rest of North America
10.1.4.1. Market Revenue and Forecast, by Product (2019-2030)
10.1.4.2. Market Revenue and Forecast, by Application (2019-2030)
10.2. Europe
10.2.1. Market Revenue and Forecast, by Product (2019-2030)
10.2.2. Market Revenue and Forecast, by Application (2019-2030)
10.2.3. UK
10.2.3.1. Market Revenue and Forecast, by Product (2019-2030)
10.2.3.2. Market Revenue and Forecast, by Application (2019-2030)
10.2.4. Germany
10.2.4.1. Market Revenue and Forecast, by Product (2019-2030)
10.2.4.2. Market Revenue and Forecast, by Application (2019-2030)
10.2.5. France
10.2.5.1. Market Revenue and Forecast, by Product (2019-2030)
10.2.5.2. Market Revenue and Forecast, by Application (2019-2030)
10.2.6. Rest of Europe
10.2.6.1. Market Revenue and Forecast, by Product (2019-2030)
10.2.6.2. Market Revenue and Forecast, by Application (2019-2030)
10.3. APAC
10.3.1. Market Revenue and Forecast, by Product (2019-2030)
10.3.2. Market Revenue and Forecast, by Application (2019-2030)
10.3.3. India
10.3.3.1. Market Revenue and Forecast, by Product (2019-2030)
10.3.3.2. Market Revenue and Forecast, by Application (2019-2030)
10.3.4. China
10.3.4.1. Market Revenue and Forecast, by Product (2019-2030)
10.3.4.2. Market Revenue and Forecast, by Application (2019-2030)
10.3.5. Japan
10.3.5.1. Market Revenue and Forecast, by Product (2019-2030)
10.3.5.2. Market Revenue and Forecast, by Application (2019-2030)
10.3.6. Rest of APAC
10.3.6.1. Market Revenue and Forecast, by Product (2019-2030)
10.3.6.2. Market Revenue and Forecast, by Application (2019-2030)
10.4. MEA
10.4.1. Market Revenue and Forecast, by Product (2019-2030)
10.4.2. Market Revenue and Forecast, by Application (2019-2030)
10.4.3. GCC
10.4.3.1. Market Revenue and Forecast, by Product (2019-2030)
10.4.3.2. Market Revenue and Forecast, by Application (2019-2030)
10.4.4. North Africa
10.4.4.1. Market Revenue and Forecast, by Product (2019-2030)
10.4.4.2. Market Revenue and Forecast, by Application (2019-2030)
10.4.5. South Africa
10.4.5.1. Market Revenue and Forecast, by Product (2019-2030)
10.4.5.2. Market Revenue and Forecast, by Application (2019-2030)
10.4.6. Rest of MEA
10.4.6.1. Market Revenue and Forecast, by Product (2019-2030)
10.4.6.2. Market Revenue and Forecast, by Application (2019-2030)
10.5. Latin America
10.5.1. Market Revenue and Forecast, by Product (2019-2030)
10.5.2. Market Revenue and Forecast, by Application (2019-2030)
10.5.3. Brazil
10.5.3.1. Market Revenue and Forecast, by Product (2019-2030)
10.5.3.2. Market Revenue and Forecast, by Application (2019-2030)
10.5.4. Rest of LATAM
10.5.4.1. Market Revenue and Forecast, by Product (2019-2030)
10.5.4.2. Market Revenue and Forecast, by Application (2019-2030)
Chapter 11. Company Profiles
11.1. Novartis
11.1.1. Company Overview
11.1.2. Product Offerings
11.1.3. Financial Performance
11.1.4. Recent Initiatives
11.2. Synthon Pharmaceuticals, Inc.
11.2.1. Company Overview
11.2.2. Product Offerings
11.2.3. Financial Performance
11.2.4. Recent Initiatives
11.3. TevaPharmaceutical Industries Ltd.
11.3.1. Company Overview
11.3.2. Product Offerings
11.3.3. Financial Performance
11.3.4. Recent Initiatives
11.4. LG Life Sciences
11.4.1. Company Overview
11.4.2. Product Offerings
11.4.3. Financial Performance
11.4.4. Recent Initiatives
11.5. Celltrion
11.5.1. Company Overview
11.5.2. Product Offerings
11.5.3. Financial Performance
11.5.4. Recent Initiatives
11.6. Biocon
11.6.1. Company Overview
11.6.2. Product Offerings
11.6.3. Financial Performance
11.6.4. Recent Initiatives
11.7. Hospira
11.7.1. Company Overview
11.7.2. Product Offerings
11.7.3. Financial Performance
11.7.4. Recent Initiatives
11.8. Merck Serono
11.8.1. Company Overview
11.8.2. Product Offerings
11.8.3. Financial Performance
11.8.4. Recent Initiatives
11.9. Biogen idec, Inc.
11.9.1. Company Overview
11.9.2. Product Offerings
11.9.3. Financial Performance
11.9.4. Recent Initiatives
11.10. Genentech
11.10.1. Company Overview
11.10.2. Product Offerings
11.10.3. Financial Performance
11.10.4. Recent Initiatives
Chapter 12. Research Methodology
12.1. Primary Research
12.2. Secondary Research
12.3. Assumptions
Chapter 13. Appendix
13.1. About Us
13.2. Glossary of Terms
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or the Asia Pacific.
Why Buy this Report?
The purpose of Precedence Research’s biosimilars market study is to provide stakeholders with a detailed picture of potential barriers and untapped opportunities. The report contains exclusive information to assist businesses in making informed decisions about how to maintain growth throughout the assessment period.
Buy Full Research Report (Single User License US$ 4500) @ https://www.precedenceresearch.com/checkout/1359
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com