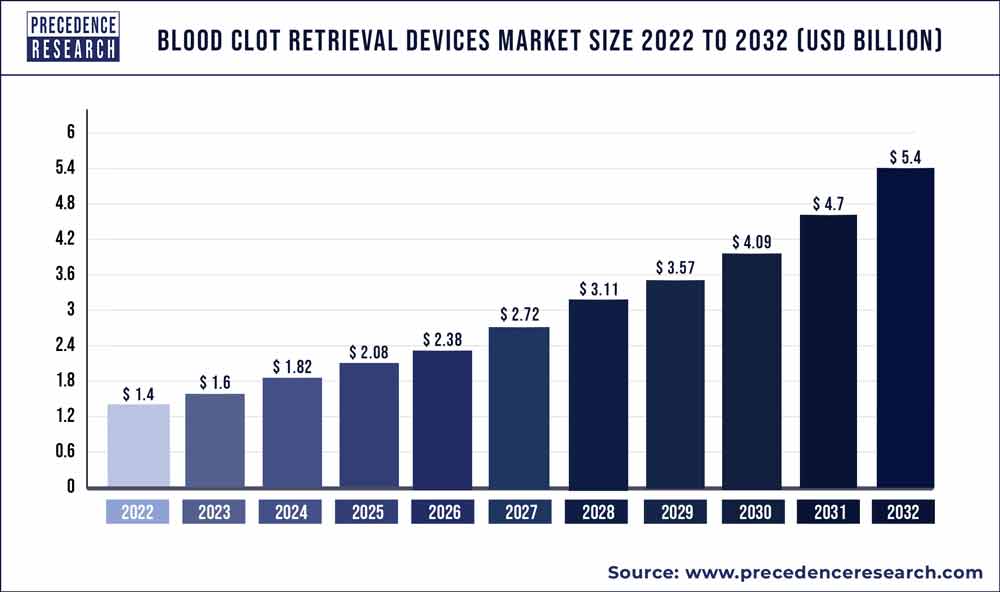
The global blood clot retrieval devices market is on the rise. According to research and consulting company, Precedence Research, the market size of blood clot retrieval devices worldwide will hit US$ 4.5 billion by 2030, up from US$ 1.49 billion in 2022. This means a cumulative average growth rate (CAGR) of 14.79% from 2022 to 2030. The report contains 150+ pages with detailed analysis.
Rise in the health conditions such as transient ischemic attack, hemorrhagic stroke and ischemia as a result of the sedentary lifestyle practices has given rise to the blood clot retrieval devices market. It is mainly or surgical process which includes the retrieval of a blood clot from the brain of the individual in order to reduce the risk of stroke disabilities, paralysis and speech disorders. The unhealthy lifestyle which is practiced by the younger generation and the geriatric age group has led to the increase in the prevalence of such grave diseases. The demand for least invasive procedures and reimbursement facilities provided by the insurance companies has helped to boost the market during the forecast period.
Blood Clot Retrieval Devices Market Regional Snapshots
As a result of the rapid development and the use of advance technology in the leading Nations such as the North America, prevalence of numerous heart and brain disorders have increased going to the sedentary and unhealthy lifestyles existing in these regions. The Asia Pacific countries such as China and India have also adopted to a number of advanced technology which has increased the sedentary lifestyle practices by the population and giving rise to increase chances of strokes and ischemic attacks among the individuals.
Download a FREE Sample Copy (Including TOC, List of Tables & Figures, and Chart) @ https://www.precedenceresearch.com/sample/1808
Report Scope of the Blood Clot Retrieval Devices Market
| Report Coverage | Details |
| Market Size by 2030 | USD 4.5 Billion |
| Growth Rate from 2022 to 2030 | CAGR of 14.79% |
| Largest Market | North America |
| Fastest Growing Region | Asia Pacific |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segments Covered | Stroke, Device, Application, End User, Geography |
Blood Clot Retrieval Devices Market Report Highlights
- By stroke type, the blood clot retrieval devices market the Ischemic segment will dominate the global blood clot retrieval devices market during the forecast. Due to an increase in the number of ischemic strokes across the globe the demand blood clot retrieval devices is expected to grow. Of all the cases of strokes repeated globally 80% are ischemic strokes.
- By end user, the hospital segment dominated the global blood clot retrieval devices market as hospitals are preferred by most of the patients. The hospital segment is expected to grow during the forecast as the interventional processes are carried out in the hospitals. Due to the large infrastructure of the hospitals with the availability of all the facilities under one roof the segment is expected to grow during the forecast period.
- By geography, the Asia Pacific region is expected to dominate the blood clot retrieval devices market in the coming years. Due to an investment for development of healthcare infrastructure across many countries in the Asia Pacific region the market is expected to grow in this region. The availability of the devices at lower cost as compared to the other countries or regions will provide opportunity for the growth of the market.
Blood Clot Retrieval Devices Market Dynamics
Drivers
The use of developed medical products used for inserting urine catheters has made the process easier and quicker. The increase in the geriatric population has increased the number of people suffering with cardiac and neurological disorders which mostly consist of clots and blockages. These disorders are treatable with the minimum invasive procedures which are done with the help of clot retrieval equipments. This helps to reduce the number of side effects which the patient has to suffer during the post operative period. Hence the life expectancy of the people belonging to the geriatric age group has also seen a steady growth in recent years. These multiple factors which provide better healthcare facilities to the population helps the market to record a significant growth during the forecast period.
Restraints
The use of blood clot retrieval equipments includes the use of highly advanced technologies that help to perform complete clot removal with the help of surgical procedures. In order to perform surgical procedures with the help of these advanced equipments highly skilled and experienced individuals are required. The lack of skilled and trained employee for the operation of these advanced equipments proves to be a restraining factor for the growth of the market during the forecast period. A few surgical procedures which fail due to the mechanical faults or conditional errors hampers the confidence that the population might show in these equipments. Hence it hampers the growth of the market worldwide. The malfunctioning of the equipment after installation of the same in the human body increases the total suffering of the patients by way of reopening of the treated areas and thus doubling the expenses.
Also Read: Smart Helmet Market Worth Nearly US$ 1537.9 Mn by 2030
Opportunities
The outbreak of the pandemic has made the people aware about healthcare facilities and health practices that are beneficial for the body. Hence people have shown a drastic shift to minimally invasive surgical techniques rather than big and long procedures that have greater side effects and take a longer period of time to heal. The high number of people belonging to the geriatric age group has propelled the number of people suffering with chronic and complex cardiovascular diseases, neurological disorders and vascular impairments. Hence the use of blood clot retrieval equipments has seen a surge during the recent times.
Challenges
The high cost which needs to be paid for these advanced technologies for the removal of blood clots from the clogged vascular systems challenges the growth of the market during the forecast period. The countries with lower socioeconomic background and which have not yet developed cannot afford these equipment and surgical procedures. My function of the blood driving machinery during the course of a procedure is still a challenge in front of the market which needs to your come and the earliest to maintain the trust on this new technology. In the event of a failure of the procedure, a second surgery needs to be performed to make things perfect.
Research Methodology
A unique research methodology has been utilized to conduct comprehensive research on the growth of the global blood clot retrieval devices market and arrive at conclusions on the future growth prospects of the market. This research methodology is a combination of primary and secondary research, which helps analysts warrant the accuracy and reliability of the draw conclusions.
Secondary sources referred to by analysts during the production of the global market report include statistics from company annual reports, SEC filings, company websites, World Bank database, investor presentations, regulatory databases, government publications, and industry white papers. Analysts have also interviewed senior managers, product portfolio managers, CEOs, VPs, and market intelligence managers, who contributed to the production of our study on the market as a primary source.
These primary and secondary sources provided exclusive information during interviews, which serves as validation from mattress topper industry leaders. Access to an extensive internal repository and external proprietary databases allows this report to address specific details and questions about the global blood clot retrieval devices market with accuracy. The study also uses the top-down approach to assess the numbers for each segment and the bottom-up approach to counter-validate them. This has helped to estimate the future prospects of the global market more reliable and accurately.
Recent Developments
- A recently developed stent retrieval machine is developed by the Cerenovus Co., which is global manufacturer of medical equipment. It is known as the Embotrap 2, which removes the life threatening blood clots from the brain resulting in ischemic stroke. It has received it’s clearance from the FDA (US) which will help the company to develop its product value and sales. This took place in 2018.
- In 2021, TigerRetriever was launched by Rapid Medical. It is a huge manufacture of responsive, adjustable devices relating to the neurovascular system. The main role of the device is to revascularise the channels during the procedures for ischemic stroke.
- In 2021, a patient was treated by ENVI .SR mechanical thrombectomy system by the NeuroVasc technologies. It is a universal medical device manufacturer. This product was used as a stent retriever which was made by the company. It was mainly used for removing blood clots.
Key Market Players
- AngioDynamics
- Terumo Co.
- Johnson and Johnson
- ECKOS Co.
- Bayer HealthCare LLC
- Boston Scientific Co.
- Argon Medical Devices
- Medtronic Plc
- Teleflex Incorporated
- Penumbra
Segments covered in the report
By Stroke
- Ischemic Stroke (blood clot)
- Hemorrhagic Stroke (rupturing of arteries)
- Transient Ischemic Attack
By Device
- Mechanical Embolus Removal Devices
- Penumbra Blood Clot Retrieval Devices
- Stent Retrievers
- Aspiration Device
- Ultrasound Assisted Devices
By Application
- Coronary Arteries
- Peripheral Arteries
- Cerebral Arteries
By End User
- Hospitals
- Diagnostic Centers
- Clinics
- Ambulatory Surgical Centers
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia-Pacific
- China
- India
- Japan
- South Korea
- Malaysia
- Philippines
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa (MEA)
- GCC
- North Africa
- South Africa
- Rest of the Middle East & Africa
Why should you invest in this report?
If you are aiming to enter the global blood clot retrieval devices market, this report is a comprehensive guide that provides crystal clear insights into this niche market. All the major application areas for blood clot retrieval devices are covered in this report and information is given on the important regions of the world where this market is likely to boom during the forecast period of 2022-2030 so that you can plan your strategies to enter this market accordingly.
Besides, through this report, you can have a complete grasp of the level of competition you will be facing in this hugely competitive market and if you are an established player in this market already, this report will help you gauge the strategies that your competitors have adopted to stay as market leaders in this market. For new entrants to this market, the voluminous data provided in this report is invaluable.
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Blood Clot Retrieval Devices Market
5.1. COVID-19 Landscape: Blood Clot Retrieval Devices Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Blood Clot Retrieval Devices Market, By Stroke
8.1. Blood Clot Retrieval Devices Market, by Stroke, 2022-2030
8.1.1. Ischemic Stroke (blood clot)
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Hemorrhagic Stroke (rupturing of arteries)
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Transient Ischemic Attack
8.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Blood Clot Retrieval Devices Market, By Devices
9.1. Blood Clot Retrieval Devices Market, by Devices, 2022-2030
9.1.1. Mechanical Embolus Removal Devices
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Penumbra Blood Clot Retrieval Devices
9.1.2.1. Market Revenue and Forecast (2017-2030)
9.1.3. Stent Retrievers
9.1.3.1. Market Revenue and Forecast (2017-2030)
9.1.4. Aspiration Device
9.1.4.1. Market Revenue and Forecast (2017-2030)
9.1.5. Ultrasound Assisted Devices
9.1.5.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Blood Clot Retrieval Devices Market, By Application
10.1. Blood Clot Retrieval Devices Market, by Application, 2022-2030
10.1.1. Coronary Arteries
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Peripheral Arteries
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Cerebral Arteries
10.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Blood Clot Retrieval Devices Market, By End User
11.1. Blood Clot Retrieval Devices Market, by End User, 2022-2030
11.1.1. Hospitals
11.1.1.1. Market Revenue and Forecast (2017-2030)
11.1.2. Diagnostic Centers
11.1.2.1. Market Revenue and Forecast (2017-2030)
11.1.3. Clinics
11.1.3.1. Market Revenue and Forecast (2017-2030)
11.1.4. Ambulatory Surgical Centers
11.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 12. Global Blood Clot Retrieval Devices Market, Regional Estimates and Trend Forecast
12.1. North America
12.1.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.1.2. Market Revenue and Forecast, by Devices (2017-2030)
12.1.3. Market Revenue and Forecast, by Application (2017-2030)
12.1.4. Market Revenue and Forecast, by End User (2017-2030)
12.1.5. U.S.
12.1.5.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.1.5.2. Market Revenue and Forecast, by Devices (2017-2030)
12.1.5.3. Market Revenue and Forecast, by Application (2017-2030)
12.1.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.1.6. Rest of North America
12.1.6.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.1.6.2. Market Revenue and Forecast, by Devices (2017-2030)
12.1.6.3. Market Revenue and Forecast, by Application (2017-2030)
12.1.6.4. Market Revenue and Forecast, by End User (2017-2030)
12.2. Europe
12.2.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.2.2. Market Revenue and Forecast, by Devices (2017-2030)
12.2.3. Market Revenue and Forecast, by Application (2017-2030)
12.2.4. Market Revenue and Forecast, by End User (2017-2030)
12.2.5. UK
12.2.5.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.2.5.2. Market Revenue and Forecast, by Devices (2017-2030)
12.2.5.3. Market Revenue and Forecast, by Application (2017-2030)
12.2.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.2.6. Germany
12.2.6.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.2.6.2. Market Revenue and Forecast, by Devices (2017-2030)
12.2.6.3. Market Revenue and Forecast, by Application (2017-2030)
12.2.6.4. Market Revenue and Forecast, by End User (2017-2030)
12.2.7. France
12.2.7.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.2.7.2. Market Revenue and Forecast, by Devices (2017-2030)
12.2.7.3. Market Revenue and Forecast, by Application (2017-2030)
12.2.7.4. Market Revenue and Forecast, by End User (2017-2030)
12.2.8. Rest of Europe
12.2.8.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.2.8.2. Market Revenue and Forecast, by Devices (2017-2030)
12.2.8.3. Market Revenue and Forecast, by Application (2017-2030)
12.2.8.4. Market Revenue and Forecast, by End User (2017-2030)
12.3. APAC
12.3.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.3.2. Market Revenue and Forecast, by Devices (2017-2030)
12.3.3. Market Revenue and Forecast, by Application (2017-2030)
12.3.4. Market Revenue and Forecast, by End User (2017-2030)
12.3.5. India
12.3.5.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.3.5.2. Market Revenue and Forecast, by Devices (2017-2030)
12.3.5.3. Market Revenue and Forecast, by Application (2017-2030)
12.3.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.3.6. China
12.3.6.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.3.6.2. Market Revenue and Forecast, by Devices (2017-2030)
12.3.6.3. Market Revenue and Forecast, by Application (2017-2030)
12.3.6.4. Market Revenue and Forecast, by End User (2017-2030)
12.3.7. Japan
12.3.7.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.3.7.2. Market Revenue and Forecast, by Devices (2017-2030)
12.3.7.3. Market Revenue and Forecast, by Application (2017-2030)
12.3.7.4. Market Revenue and Forecast, by End User (2017-2030)
12.3.8. Rest of APAC
12.3.8.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.3.8.2. Market Revenue and Forecast, by Devices (2017-2030)
12.3.8.3. Market Revenue and Forecast, by Application (2017-2030)
12.3.8.4. Market Revenue and Forecast, by End User (2017-2030)
12.4. MEA
12.4.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.4.2. Market Revenue and Forecast, by Devices (2017-2030)
12.4.3. Market Revenue and Forecast, by Application (2017-2030)
12.4.4. Market Revenue and Forecast, by End User (2017-2030)
12.4.5. GCC
12.4.5.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.4.5.2. Market Revenue and Forecast, by Devices (2017-2030)
12.4.5.3. Market Revenue and Forecast, by Application (2017-2030)
12.4.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.4.6. North Africa
12.4.6.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.4.6.2. Market Revenue and Forecast, by Devices (2017-2030)
12.4.6.3. Market Revenue and Forecast, by Application (2017-2030)
12.4.6.4. Market Revenue and Forecast, by End User (2017-2030)
12.4.7. South Africa
12.4.7.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.4.7.2. Market Revenue and Forecast, by Devices (2017-2030)
12.4.7.3. Market Revenue and Forecast, by Application (2017-2030)
12.4.7.4. Market Revenue and Forecast, by End User (2017-2030)
12.4.8. Rest of MEA
12.4.8.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.4.8.2. Market Revenue and Forecast, by Devices (2017-2030)
12.4.8.3. Market Revenue and Forecast, by Application (2017-2030)
12.4.8.4. Market Revenue and Forecast, by End User (2017-2030)
12.5. Latin America
12.5.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.5.2. Market Revenue and Forecast, by Devices (2017-2030)
12.5.3. Market Revenue and Forecast, by Application (2017-2030)
12.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.5.5. Brazil
12.5.5.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.5.5.2. Market Revenue and Forecast, by Devices (2017-2030)
12.5.5.3. Market Revenue and Forecast, by Application (2017-2030)
12.5.5.4. Market Revenue and Forecast, by End User (2017-2030)
12.5.6. Rest of LATAM
12.5.6.1. Market Revenue and Forecast, by Stroke (2017-2030)
12.5.6.2. Market Revenue and Forecast, by Devices (2017-2030)
12.5.6.3. Market Revenue and Forecast, by Application (2017-2030)
12.5.6.4. Market Revenue and Forecast, by End User (2017-2030)
Chapter 13. Company Profiles
13.1. AngioDynamics
13.1.1. Company Overview
13.1.2. Product Offerings
13.1.3. Financial Performance
13.1.4. Recent Initiatives
13.2. Terumo Co.
13.2.1. Company Overview
13.2.2. Product Offerings
13.2.3. Financial Performance
13.2.4. Recent Initiatives
13.3. Johnson and Johnson
13.3.1. Company Overview
13.3.2. Product Offerings
13.3.3. Financial Performance
13.3.4. Recent Initiatives
13.4. ECKOS Co.
13.4.1. Company Overview
13.4.2. Product Offerings
13.4.3. Financial Performance
13.4.4. Recent Initiatives
13.5. Bayer HealthCare LLC
13.5.1. Company Overview
13.5.2. Product Offerings
13.5.3. Financial Performance
13.5.4. Recent Initiatives
13.6. Boston Scientific Co.
13.6.1. Company Overview
13.6.2. Product Offerings
13.6.3. Financial Performance
13.6.4. Recent Initiatives
13.7. Argon Medical Devices
13.7.1. Company Overview
13.7.2. Product Offerings
13.7.3. Financial Performance
13.7.4. Recent Initiatives
13.8. Medtronic Plc
13.8.1. Company Overview
13.8.2. Product Offerings
13.8.3. Financial Performance
13.8.4. Recent Initiatives
13.9. Teleflex Incorporated
13.9.1. Company Overview
13.9.2. Product Offerings
13.9.3. Financial Performance
13.9.4. Recent Initiatives
13.10. Penumbra
13.10.1. Company Overview
13.10.2. Product Offerings
13.10.3. Financial Performance
13.10.4. Recent Initiatives
Chapter 14. Research Methodology
14.1. Primary Research
14.2. Secondary Research
14.3. Assumptions
Chapter 15. Appendix
15.1. About Us
15.2. Glossary of Terms
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or the Asia Pacific.
Buy this Premium Research Report@ https://www.precedenceresearch.com/checkout/1808
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://precedenceresearchnews.wordpress.com
Frequently Asked Questions:
[sc_fs_multi_faq headline-0=”h3″ question-0=”Who are the major players operating in the blood clot retrieval devices market?” answer-0=”The major players operating in the blood clot retrieval devices market are AngioDynamics, Terumo Co., Johnson and Johnson, ECKOS Co., Bayer HealthCare LLC, Boston Scientific Co., Argon Medical Devices, Medtronic Plc, Teleflex Incorporated, and Penumbra. ” image-0=”” headline-1=”h3″ question-1=”What is the current size of blood clot retrieval devices market?” answer-1=”The global blood clot retrieval devices market size was reached at US$ 1.3 billion in 2021 and it is anticipated to rake around US$ 4.5 billion by 2030. ” image-1=”” headline-2=”h3″ question-2=”What will be the CAGR of global blood clot retrieval devices market?” answer-2=”The global blood clot retrieval devices market is growing at a CAGR of 14.79% from year 2022 to 2030. ” image-2=”” headline-3=”h3″ question-3=”Which are the driving factors of the fruit and vegetable ingredients market?” answer-3=”Increasing geriatric population across all the developed as well as developing nations is expected to drive the market. On an average about 8 Lac patients suffer with strokes add this number is driving the market growth. ” image-3=”” count=”4″ html=”true” css_class=””]