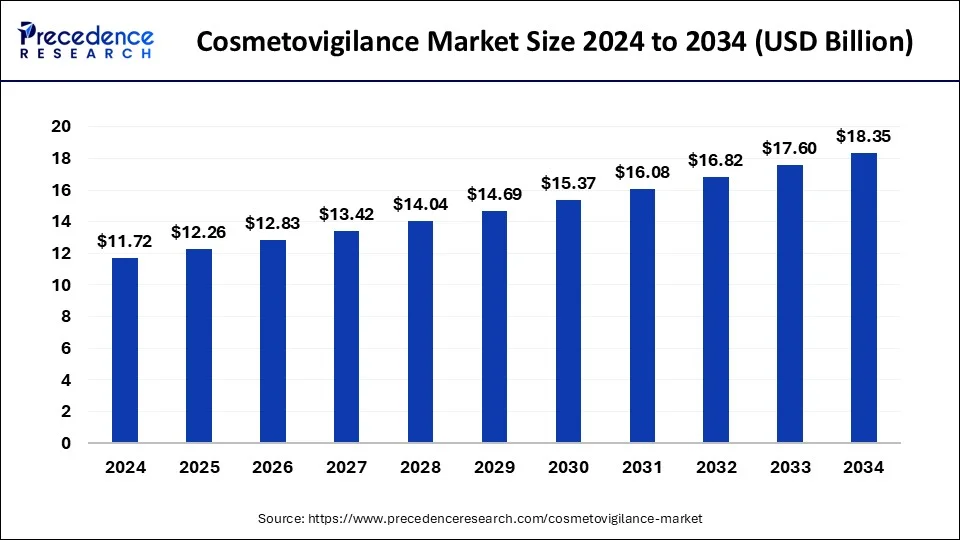
Cosmetovigilance Market Size to Reach USD 17.60 Bn by 2033
The global cosmetovigilance market size surpassed USD 11.20 billion in 2023 and is expected to be worth around USD 17.60 billion by 2033, growing at a CAGR of 4.62% from 2024 to 2033.
Key Points
- Europe has captured highest revenue share in cosmetovigilance market in 2023 with 37.8%.
- By service type, the post-marketing services market segment accounted for the dominant share of the market in 2023 with 64%.
- By service type, the pre-marketing services segment is expected to witness considerable growth in the global market over the forecast period.
- By categories, the skincare segment held the largest share of around 33% in 2023.
- By service provider, the contract outsourcing segment has accounted largest revenue share of 60% in 2023.
- By phase type, the Phase IV segment has held revenue share of 76% in 2023.
Cosmetovigilance refers to the monitoring and reporting of adverse effects or incidents related to cosmetic products. It involves the systematic collection, analysis, and evaluation of safety data to ensure the continued safety and efficacy of cosmetics in the market. With the growing demand for cosmetic products globally, the importance of cosmetovigilance has increased significantly to safeguard consumer health and well-being. This article provides an overview of the cosmetovigilance market, including its growth factors, regional insights, drivers, opportunities, and challenges.
Get a Sample: https://www.precedenceresearch.com/sample/4062
Growth Factors:
The cosmetovigilance market is primarily driven by the increasing awareness among consumers regarding the safety and quality of cosmetic products. With rising concerns about potential adverse effects from cosmetics, consumers are becoming more vigilant about the ingredients used in their personal care products. This has led to a greater demand for stringent regulatory measures and surveillance systems to monitor the safety of cosmetics and ensure compliance with regulatory standards.
Moreover, the globalization of the cosmetic industry and the proliferation of online sales channels have expanded the market reach of cosmetic products, leading to greater exposure and potential risks. As a result, regulatory authorities and manufacturers are increasingly investing in cosmetovigilance activities to detect, assess, and mitigate safety concerns associated with cosmetics across different markets and regions.
Region Insights:
The cosmetovigilance market exhibits varying dynamics across different regions, influenced by factors such as regulatory frameworks, consumer behavior, and market maturity.
In North America, stringent regulatory requirements by agencies such as the U.S. Food and Drug Administration (FDA) and Health Canada drive the demand for robust cosmetovigilance systems. The region also benefits from a mature cosmetics market and a highly informed consumer base, contributing to the adoption of cosmetovigilance practices by manufacturers and regulatory authorities.
In Europe, the European Union’s stringent regulations under the Cosmetic Product Regulation (CPR) mandate rigorous safety assessments and reporting obligations for cosmetic products. The presence of key players in the cosmetics industry and growing consumer awareness further bolster the cosmetovigilance market in the region.
In Asia-Pacific, rapid urbanization, rising disposable incomes, and changing consumer preferences are driving the demand for cosmetic products. However, the regulatory landscape for cosmetovigilance in many Asian countries is still evolving, presenting both opportunities and challenges for market players.
Cosmetovigilance Market Scope
| Report Coverage | Details |
| Global Market Size in 2023 | USD 11.20 Billion |
| Global Market Size in 2024 | USD 11.72 Billion |
| Global Market Size by 2033 | USD 17.60 Billion |
| Growth Rate from 2024 to 2033 | CAGR of 4.62% |
| Largest Market | Europe |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | By Service Type, By Categories, By Phase Type, and By Service Provider |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Cosmetovigilance Market Dynamics
Drivers:
Several factors drive the growth of the cosmetovigilance market. One of the primary drivers is the increasing focus on consumer safety and regulatory compliance by cosmetic manufacturers and regulatory authorities. Stringent regulatory requirements, along with the growing consumer demand for transparency and accountability, compel companies to invest in cosmetovigilance activities to ensure the safety and quality of their products.
Moreover, advancements in technology, such as data analytics, artificial intelligence, and digital reporting systems, are enhancing the efficiency and effectiveness of cosmetovigilance processes. Automated data collection, signal detection algorithms, and real-time monitoring capabilities enable timely identification and response to safety concerns, reducing the risk of adverse events associated with cosmetic products.
Opportunities:
The cosmetovigilance market presents several opportunities for growth and innovation. One such opportunity lies in leveraging technology to enhance pharmacovigilance practices in the cosmetic industry. By adopting digital platforms, mobile applications, and data analytics tools, companies can streamline adverse event reporting, improve data quality, and enhance communication with consumers and regulatory authorities.
Furthermore, collaboration and knowledge sharing among stakeholders, including manufacturers, regulatory agencies, healthcare professionals, and consumer advocacy groups, can strengthen cosmetovigilance efforts and promote a culture of safety and transparency in the cosmetics industry. Training programs, workshops, and conferences on cosmetovigilance best practices can help build capacity and expertise within the industry.
Challenges:
Despite the opportunities, the cosmetovigilance market faces several challenges that must be addressed to ensure its effectiveness and sustainability. One of the primary challenges is the lack of harmonization and consistency in regulatory requirements and reporting standards across different regions and jurisdictions. This fragmentation complicates compliance efforts for multinational companies and hinders the sharing of safety data and best practices.
Moreover, underreporting of adverse events, inadequate resources, and limited awareness among healthcare professionals and consumers pose challenges to the timely detection and assessment of safety concerns associated with cosmetic products. Addressing these challenges requires concerted efforts by regulatory authorities, industry stakeholders, and healthcare providers to promote transparency, education, and collaboration in cosmetovigilance activities.
Read Also: IT Services Market Size to Surpass USD 2.80 Trillion By 2033
Recent Developments
- In December 2023, Biofrontera AG, an international biopharmaceutical company, announced that its wholly-owned subsidiary Biofrontera Bioscience GmbH has received a notice of allowance for the application “Photodynamic therapy comprising two light exposures at different wavelengths by the US patent office USPTO. This patent protects several innovations relating to a new illumination method to treat dermatological skin diseases with Photodynamic Therapy (PDT).
- In June 2023, Anju Software and ClinChoice partnered to enhance eClinical solutions for clinical research. ClinChoice’s acquisition of Cromsource led to the collaboration, enabling the delivery of adaptable and cost-efficient eClinical solutions, including TrialMaster, for streamlined clinical trial data management and regulatory compliance.
Cosmetovigilance Market Companies
- Poseidon CRO
- AxeRegel
- PharSafer
- AB Cube
- Aixial Group
- Di Renzo
- Pharmathen
- Skill Pharma
- OC Vigilance
- Preclinical
- safety
- ZEINCRO Group
- FMD K&L
- POSEIDON CRO
- MSL Solution Providers
- Cliantha
- Pure Drug Safety
- KMJ pharma sp. z o.o.
- TheraSkin
- CORONIS Research SA
- PHARMALEX GMBH
- Bioclinica
- Tecnimede Group
- Sciformix
Segments Covered in the Report
By Service Type
- Pre-marketing Services
- Clinical Safety Testing
- Document Writing
- Risk Management
- Post-marketing Services
- Case Intake
- Case Triage
- Data Entry & Acquisition
- Tracking & Reporting
By Categories
- Skincare
- Makeup
- Haircare
- Perfumes and deodorants
- Hair colorants
- Others
By Phase Type
- Pre-clinical
- Phase I
- Phase II
- Phase III
- Phase IV
By Service Provider
- In-house
- Contract Outsourcing
- Contract Research Organizations (CROs)
- Business Process Outsourcing Organizations (BPOs)
By Geography
- North America
- Europe
- Asia-Pacific
- Latin America
- Middle East and Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/