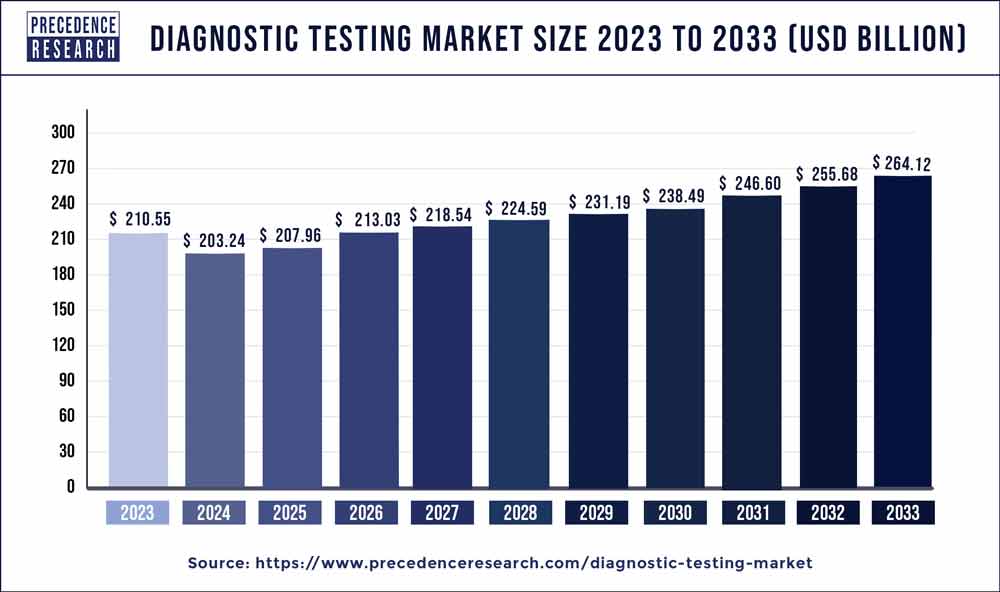
Diagnostic Testing Market Revenue Expected to Reach $264.12 Bn by 2033
[150+ Pages Report] The global diagnostic testing market revenue was 210.55 billion in 2023 and is anticipated to reach around USD 264.12 billion by 2033, with a CAGR of 3% over the forecast period of 2024 to 2033. The growth of the market has been significantly influenced by the rising demand for point-of-care clinical diagnostics devices and the growing use of these devices.
The Diagnostic Testing Industry has undergone a significant transformation in recent years, reshaping the landscape of modern medicine. This evolution stems from advancements in technology, research, and the growing demand for precision healthcare solutions. As a result, diagnostic testing has emerged as a cornerstone in the diagnosis, treatment, and prevention of various medical conditions.
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/1984
Diagnostic testing plays a pivotal role in healthcare by providing vital insights into an individual’s health status. From routine check-ups to complex disease diagnoses, these tests offer healthcare professionals valuable information to make informed decisions about patient care. With advancements in medical imaging, genetic testing, and biomarker analysis, diagnostic tests have become increasingly accurate, efficient, and accessible.
In recent years, the diagnostic testing industry has witnessed a surge in technological innovations, fueling advancements in precision medicine and personalized healthcare. Next-generation sequencing (NGS), liquid biopsy, and point-of-care testing are just a few examples of cutting-edge technologies revolutionizing diagnostics. These innovations enable early detection of diseases, tailored treatment approaches, and improved patient outcomes.
The evolution of diagnostic testing has transformed the way diseases are managed and treated. Through early detection and accurate diagnosis, healthcare professionals can intervene promptly, potentially preventing disease progression and complications. Furthermore, companion diagnostics have emerged as indispensable tools in guiding targeted therapies, ensuring patients receive the most effective treatments based on their unique genetic makeup and disease characteristics.
The diagnostic testing industry is instrumental in addressing global health challenges, including infectious diseases, chronic conditions, and emerging epidemics. Rapid diagnostic tests for infectious pathogens enable timely containment and management of outbreaks, reducing the spread of infections and saving lives. Moreover, advancements in cancer diagnostics contribute to early detection and improved survival rates, alleviating the burden of this devastating disease worldwide.
One of the most significant impacts of diagnostic testing is its role in enhancing patient care and outcomes. By providing healthcare professionals with accurate and timely information, diagnostic tests empower them to tailor treatment plans to individual patient needs, optimizing efficacy and minimizing adverse effects. Additionally, comprehensive diagnostic panels offer a holistic approach to healthcare, allowing for the identification of underlying health issues and risk factors before they escalate into serious conditions.
Looking ahead, the diagnostic testing industry is poised for continued growth and innovation. Technological advancements, coupled with big data analytics and artificial intelligence (AI), will further enhance the accuracy, efficiency, and accessibility of diagnostic tests. Additionally, telemedicine and remote monitoring solutions will expand access to diagnostic services, particularly in underserved communities and remote regions.
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/1984
Diagnostic Testing Market Dynamics
Drivers
Growing prevalence of chronic diseases, significant R&D spending, and advancements in diagnostics systems boosting market growth for diagnostics. According to the Non-communicable Illnesses Key Facts report released by the World Health Organization (WHO) in April 2021, chronic diseases are responsible for around 41 million deaths year, or 71% of all fatalities globally. The need for healthcare systems is growing as a result of the rise in chronic illnesses. Thus, clinical diagnostics have demonstrated value in the management of chronic disease conditions as well as in the prevention, identification, and detection of disease.
Growth is partly attributable to the expanding use of clinical diagnostics, which is a result of the boost in testing brought on by the epidemic. Over the course of the study period, the market growth is anticipated to be boosted by the development of automated clinical diagnostics systems for laboratories and hospitals to give quick, accurate, and error-free diagnosis. The market is expanding due to the increasing number of clinical diagnostics products being introduced by major companies. Clinical diagnostic tools with molecular diagnostic capability produce reliable findings.
Due to the rising prevalence of infectious and chronic illnesses as well as the rising use of automated platforms, the market is predicted to expand. Government financing and the increasing number of tests due to the steady increase in patients are the two factors that are anticipated to increase demand for COVID-19 test kits and propel the market’s overall expansion significantly.
The growth of the market has been significantly influenced by the rising demand for point-of-care clinical diagnostics devices and the growing use of these devices. This is further supported by key businesses investing more money in research and development to create new products and examine novel uses for clinical diagnostics methods.
Restraints
In the last ten years, there have been considerable improvements and innovations in clinical diagnostic products, including the adoption of new products and further design changes. However, the high price of clinical diagnostics equipment and the high cost of instrument upkeep have in some ways limited the market’s expansion. Furthermore, trained people are needed to operate clinical diagnostics equipment. As a result, the maintenance cost of the gadget rises, which eventually slows market expansion.
Opportunities
Furthermore, the market participants would have lucrative prospects due to the expansion of healthcare decentralisation from 2024 to 2033. The market will also grow as a result of an increase in investments and funds for product development.
Challenges
On the other side, it is anticipated that pricing pressure brought on by reimbursement reductions, budgetary restraints, and strict regulatory rules would hinder market expansion. Additionally, insufficient adoption of Point of Care devices in professional settings and a lack of agreement with clear central lab methodologies are anticipated to provide challenges to the diagnostic tests market during the forecast period of 2024–2033.
Diagnostic Testing Market Report Scope
| Report Coverage | Details |
| Market Size in 2023 | USD 210.55 Billion |
| Market Size by 2033 | USD 264.12 Billion |
| Growth Rate from 2024 to 2033 | CAGR of 3% |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Type, Application, Approach, Solution, Technology, Mode of Testing, Sample Type, Testing Type, Age, Distribution Channel, End User, Geography |
| Companies Mentioned | F-Hoffman La-Rcohe Ltd. (Switzerland), Danaher (US), BD (US), Thermo Fisher Scientific Inc. (US), ACON Laboratories Inc. (US), Hemosure, Inc. (US), MicroGen Diagnostics (US), Grifols, S.A (Spain), BODITECH MED INC. (South Korea), Chembio Diagnostic Systems, Inc. (US), Nanoentek (South Korea), DiaSorin S.p.A. (Italy), Bio-Rad Laboratories, Inc. (US), BIOMEDOMICS INC (US), EKF Diagnostics Holdings plc (UK), Siemens Healthcare GmbH (Germany), PerkinElmer Inc. (US), bioMérieux SA (France), ARKRAY USA, Inc. (US), Biohit Oyj (Finland), Quidel Corporation (US), Illumina, Inc. (US) |
Diagnostic Testing Market Highlights
- The advent of several illnesses and infections is expected to cause the size of the worldwide market to increase to USD 339,893 million by 2030. The market for diagnostic tests is expanding at an accelerated rate due to an increase in investment.
- Hematology is predicted to lead the market on the basis of testing type because the majority of diagnoses tests use blood to find and identify disorders.
- Due to the fact that most diagnostic tests are carried out on elderly patients, the geriatric sector is predicted to dominate the market. As they are more susceptible to infectious, chronic, and non-chronic illnesses, elderly patients need frequent monitoring and diagnosis. North America now dominates the diagnostic test market as a result of the region’s growing geriatric population base.
Read Also: North America Behavioral Health Market Size, ShareReport by 2033
Diagnostic Testing Secor Trends:
- Decentralized Testing: A notable trend in the diagnostic testing market is the shift towards decentralized testing. Point-of-care diagnostics and home testing kits are gaining popularity, offering convenience and rapid results, thereby improving patient accessibility and reducing turnaround times.
- Non-Invasive Diagnostic Procedures: The market is witnessing an increasing demand for non-invasive diagnostic procedures. Technologies such as liquid biopsy and imaging modalities that eliminate the need for invasive methods are gaining traction, providing patients with alternatives that are less discomforting and pose fewer risks.
- Integration of Artificial Intelligence (AI): The incorporation of artificial intelligence in diagnostic testing is a prominent trend. AI-driven algorithms enhance data analysis, leading to improved accuracy and efficiency in diagnostics. Machine learning applications are being utilized for pattern recognition, aiding in the interpretation of complex diagnostic data.
- Digital Health Technologies: The integration of digital health technologies is reshaping the diagnostic landscape. Mobile apps, wearable devices, and telehealth platforms are increasingly being utilized for remote monitoring, data collection, and real-time communication between patients and healthcare providers, facilitating a more connected and efficient diagnostic process.
- Rapid Diagnostic Tests: The COVID-19 pandemic has accelerated the adoption of rapid diagnostic tests. The need for quick and on-the-spot results has driven innovation in point-of-care testing for various infectious diseases, contributing to the market’s responsiveness to public health emergencies.
- Personalized Medicine: The trend towards personalized medicine is influencing diagnostic testing. Molecular diagnostics and genetic testing are becoming more sophisticated, allowing for a tailored approach to treatment based on an individual’s genetic makeup, leading to more effective and targeted therapeutic interventions.
- Preventive Healthcare Emphasis: Increasing emphasis on preventive healthcare is shaping diagnostic testing trends. Early disease detection, health screening, and proactive health management are gaining prominence, driving the demand for diagnostics that support preventive care strategies.
- Global Health Awareness: Growing global awareness about health issues and the importance of diagnostics in disease prevention is fostering market growth. Campaigns, educational initiatives, and increased public knowledge contribute to a proactive approach to healthcare, driving the uptake of diagnostic testing services.
Diagnostic Testing Market Growth
The growth of the diagnostic testing market is propelled by several key factors. Firstly, the increasing prevalence of chronic diseases worldwide has intensified the demand for accurate and timely diagnostic solutions. Additionally, advancements in technology, particularly in areas such as molecular diagnostics and next-generation sequencing, contribute significantly to the market’s expansion. The rising awareness among individuals about the importance of early disease detection and preventive healthcare further fuels the demand for diagnostic testing.
Moreover, the integration of artificial intelligence for data analysis enhances diagnostic accuracy and efficiency. The ongoing global focus on public health, accentuated by the lessons learned from the COVID-19 pandemic, has accelerated the adoption of rapid diagnostic tests. As the industry continues to address these factors, the diagnostic testing market is poised for sustained growth, offering innovative solutions to meet evolving healthcare needs.
Regional Snapshots
Diagnostic Testing Market Revenue, By Region ($ Billion)
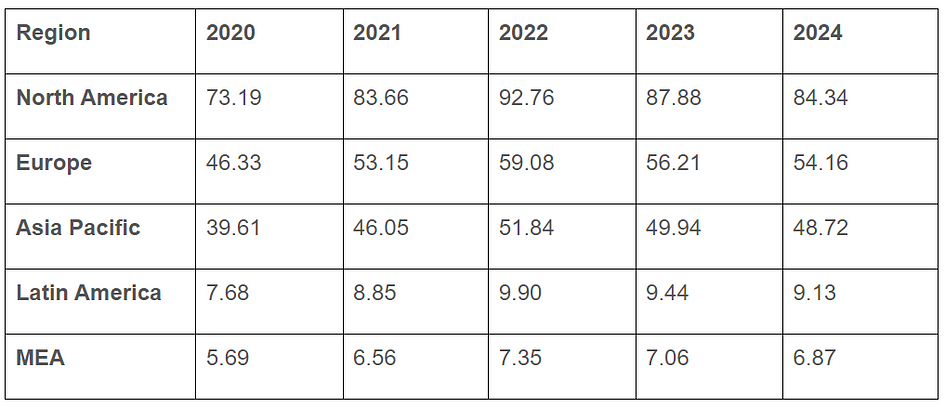
North America had the highest revenue share (more than 40%). This can be ascribed to the population’s rising health consciousness on the advantages of these tests and the region’s thriving healthcare business. Due to the fast-rising COVID-19 cases and the existence of important players in the U.S. and Canada, the area will continue its leading position throughout the projection years. For instance, bioLytical Laboratories Inc., a Canadian company, gained CE certification for the iStatis COVID-19 Antigen Home Test in March 2022. The business will be able to reach the European market thanks to this.
The Asia Pacific market is anticipated to develop at the quickest rate among all regions. A growing number of regional producers of diagnostic kits and reagents are anticipated to propel the point-of-care market in the Asia Pacific, providing a variety of testing options for the detection of coronavirus infection. In order to increase COVID-19 testing, the nations are continually expanding their capabilities. For example, the Malaysian Ministry of Health approved the use of antigen quick testing kits shipped from South Korea in April 2020 to expand the nation’s COVID-19 testing to a daily capacity of 16,500 tests.
Key Market Developments
- In January 2021, Roche introduced its Cobas Pulse System in a few nations that accepted the CE Mark. The item is a networked point-of-care solution from Roche Diagnostics’ most recent generation. The most recent release provides expert blood glucose management.
- In September 2021, Ortho Clinical Diagnostics announced that Immediate Spin Crossmatch (ISXM), a tool for identifying recipient and donor incompatibility in blood transfusions, will be made available on the company’s Ortho Vision and Ortho Vision Max Analyzers.
Diagnostic Testing Market Players
- F-Hoffman La-Rcohe Ltd. (Switzerland)
- Danaher (US)
- BD (US)
- Thermo Fisher Scientific Inc. (US)
- ACON Laboratories Inc. (US)
- Hemosure, Inc. (US)
- MicroGen Diagnostics (US)
- Grifols, S.A (Spain)
- BODITECH MED INC. (South Korea)
- Chembio Diagnostic Systems, Inc. (US)
- Nanoentek (South Korea)
- DiaSorin S.p.A. (Italy)
- Bio-Rad Laboratories, Inc. (US)
- BIOMEDOMICS INC (US)
- EKF Diagnostics Holdings plc (UK)
- Siemens Healthcare GmbH (Germany)
- PerkinElmer Inc. (US)
- bioMérieux SA (France)
- ARKRAY USA, Inc. (US)
- Biohit Oyj (Finland)
- Quidel Corporation (US)
- Illumina, Inc. (US)
- Lamdagen Corporation (US)
- LifeSign LLC. (US)
- Medixbiochemica (Finaland)
- Nova Biomedical (US)
- Ortho Clinical Diagnostics (US)
- Sannuo Biosensing Co., Ltd. (US)
- STRECK (US)
- Sysmex Corporation (Japan)
Segments covered in the report
By Type
- Clinical Diagnostic
- Home Diagnostic
By Application
- Cardiology
- Oncology
- Neurology
- Orthopedics
- Gastroenterology
- Gynecology
- Odontology
- Others
By Approach
- Molecular Diagnostic Instrument
- In-Vitro Diagnostic Instrument
- Point Of Care Testing Instrument
By Solution
- Services
- Products
By Technology
- Immunoassay-Based
- PCR-Based
- Next-generation Sequencing
- Spectroscopy-Based
- Chromatography-Based
- Microfluidics
- Substrate Technology
- Others
By Mode of Testing
- Prescription Based Testing
- OTC Testing
By Sample Type
- Urine
- Saliva
- Blood
- Hair
- Sweat
- Others
By Testing Type
- Biochemistry
- Hematology
- Microbiology
- Histopathology
- Others
By Age
- Pediatric
- Adult & Geriatric
By Distribution Channel
- Direct Tenders
- Retail Sales
- Online Sales
By End User
- Hospitals, Diagnostic Center
- Research Labs and Institutes
- Research Institute
- Homecare
- Blood Banks
- Specialty Clinics
- Ambulatory Surgical Centers
- Others
By Geography
- North America
- U.S.
- Canada
- Europe
- U.K.
- Germany
- France
- Asia-Pacific
- China
- India
- Japan
- South Korea
- Malaysia
- Philippines
- Latin America
- Brazil
- Rest of Latin America
- Middle East & Africa (MEA)
- GCC
- North Africa
- South Africa
- Rest of the Middle East & Africa
Immediate Delivery Available | Buy This Premium Research Report@ https://www.precedenceresearch.com/checkout/1984
Table Of Contents
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Ages
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Eleven Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. Market Dynamics Analysis and Trends
5.1. Market Dynamics
5.1.1. Market Drivers
5.1.2. Market Restraints
5.1.3. Market Opportunities
5.2. Porter’s Five Forces Analysis
5.2.1. Bargaining power of suppliers
5.2.2. Bargaining power of buyers
5.2.3. Threat of substitute
5.2.4. Threat of new entrants
5.2.5. Degree of competition
Chapter 6. Competitive Landscape
6.1.1. Company Market Share/Positioning Analysis
6.1.2. Key Strategies Adopted by Players
6.1.3. Vendor Landscape
6.1.3.1. List of Suppliers
6.1.3.2. List of Buyers
Chapter 7. Global Diagnostic Testing Market, By Type
7.1. Diagnostic Testing Market, by Type, 2024-2033
7.1.1. Clinical Diagnostic
7.1.1.1. Market Revenue and Forecast (2020-2033)
7.1.2. Home Diagnostic
7.1.2.1. Market Revenue and Forecast (2020-2033)
Chapter 8. Global Diagnostic Testing Market, By Application
8.1. Diagnostic Testing Market, by Application, 2024-2033
8.1.1. Cardiology
8.1.1.1. Market Revenue and Forecast (2020-2033)
8.1.2. Oncology
8.1.2.1. Market Revenue and Forecast (2020-2033)
8.1.3. Neurology
8.1.3.1. Market Revenue and Forecast (2020-2033)
8.1.4. Orthopedics
8.1.4.1. Market Revenue and Forecast (2020-2033)
8.1.5. Gastroenterology
8.1.5.1. Market Revenue and Forecast (2020-2033)
8.1.6. Gynecology
8.1.6.1. Market Revenue and Forecast (2020-2033)
8.1.7. Odontology
8.1.8.1. Market Revenue and Forecast (2020-2033)
8.1.9. Others
8.1.9.1. Market Revenue and Forecast (2020-2033)
Chapter 9. Global Diagnostic Testing Market, By Approach
9.1. Diagnostic Testing Market, by Approach, 2024-2033
9.1.1. Molecular Diagnostic Instrument
9.1.1.1. Market Revenue and Forecast (2020-2033)
9.1.2. In-Vitro Diagnostic Instrument
9.1.2.1. Market Revenue and Forecast (2020-2033)
9.1.3. Point Of Care Testing Instrument
9.1.3.1. Market Revenue and Forecast (2020-2033)
Chapter 10. Global Diagnostic Testing Market, By Solution
10.1. Diagnostic Testing Market, by Solution, 2024-2033
10.1.1. Services
10.1.1.1. Market Revenue and Forecast (2020-2033)
10.1.2. Products
10.1.2.1. Market Revenue and Forecast (2020-2033)
Chapter 11. Global Diagnostic Testing Market, By Technology
11.1. Diagnostic Testing Market, by Technology, 2024-2033
11.1.1. Immunoassay-Based
11.1.1.1. Market Revenue and Forecast (2020-2033)
11.1.2. PCR-Based
11.1.2.1. Market Revenue and Forecast (2020-2033)
11.1.3. Next-generation Sequencing
11.1.3.1. Market Revenue and Forecast (2020-2033)
11.1.4. Spectroscopy-Based
11.1.4.1. Market Revenue and Forecast (2020-2033)
11.1.5. Chromatography-Based
11.1.5.1. Market Revenue and Forecast (2020-2033)
11.1.6. Microfluidics
11.1.6.1. Market Revenue and Forecast (2020-2033)
11.1.7. Substrate Technology
11.1.7.1. Market Revenue and Forecast (2020-2033)
11.1.8. Others
11.1.8.1. Market Revenue and Forecast (2020-2033)
Chapter 12. Global Diagnostic Testing Market, By Mode of Testing
12.1. Diagnostic Testing Market, by Mode of Testing, 2024-2033
12.1.1. Prescription Based Testing
12.1.1.1. Market Revenue and Forecast (2020-2033)
12.1.2. OTC Testing
12.1.2.1. Market Revenue and Forecast (2020-2033)
Chapter 13. Global Diagnostic Testing Market, By Sample Type
13.1. Diagnostic Testing Market, by Sample Type, 2024-2033
13.1.1. Urine
13.1.1.1. Market Revenue and Forecast (2020-2033)
13.1.2. Saliva
13.1.2.1. Market Revenue and Forecast (2020-2033)
13.1.3. Blood
13.1.3.1. Market Revenue and Forecast (2020-2033)
13.1.4. Hair
13.1.4.1. Market Revenue and Forecast (2020-2033)
13.1.5. Sweat
13.1.5.1. Market Revenue and Forecast (2020-2033)
13.1.6. Others
13.1.6.1. Market Revenue and Forecast (2020-2033)
Chapter 14. Global Diagnostic Testing Market, By Testing Type
14.1. Diagnostic Testing Market, by Testing Type, 2024-2033
14.1.1. Biochemistry
14.1.1.1. Market Revenue and Forecast (2020-2033)
14.1.2. Hematology
14.1.2.1. Market Revenue and Forecast (2020-2033)
14.1.3. Microbiology
14.1.3.1. Market Revenue and Forecast (2020-2033)
14.1.4. Histopathology
14.1.4.1. Market Revenue and Forecast (2020-2033)
14.1.5. Others
14.1.5.1. Market Revenue and Forecast (2020-2033)
Chapter 15. Global Diagnostic Testing Market, By Age
15.1. Diagnostic Testing Market, by Age, 2024-2033
15.1.1. Pediatric
15.1.1.1. Market Revenue and Forecast (2020-2033)
15.1.2. Adult & Geriatric
15.1.2.1. Market Revenue and Forecast (2020-2033)
Chapter 16. Global Diagnostic Testing Market, By Distribution Channel
16.1. Diagnostic Testing Market, by Distribution Channel, 2024-2033
16.1.1. Direct Tenders
16.1.1.1. Market Revenue and Forecast (2020-2033)
16.1.2. Retail Sales
16.1.2.1. Market Revenue and Forecast (2020-2033)
16.1.3. Online Sales
16.1.3.1. Market Revenue and Forecast (2020-2033)
Chapter 17. Global Diagnostic Testing Market, By End User
17.1. Diagnostic Testing Market, by End User, 2024-2033
17.1.1. Hospitals, Diagnostic Center
17.1.1.1. Market Revenue and Forecast (2020-2033)
17.1.2. Research Labs and Institutes
17.1.2.1. Market Revenue and Forecast (2020-2033)
17.1.3. Research Institute
17.1.3.1. Market Revenue and Forecast (2020-2033)
17.1.4. Homecare
17.1.4.1. Market Revenue and Forecast (2020-2033)
17.1.5. Blood Banks
17.1.5.1. Market Revenue and Forecast (2020-2033)
17.1.6. Specialty Clinics
17.1.6.1. Market Revenue and Forecast (2020-2033)
17.1.7. Ambulatory Surgical Centers
17.1.7.1. Market Revenue and Forecast (2020-2033)
17.1.8. Others
17.1.8.1. Market Revenue and Forecast (2020-2033)
Chapter 18. Global Diagnostic Testing Market, Regional Estimates and Trend Forecast
18.1. North America
18.1.1. Market Revenue and Forecast, by Type (2020-2033)
18.1.2. Market Revenue and Forecast, by Application (2020-2033)
18.1.3. Market Revenue and Forecast, by Approach (2020-2033)
18.1.4. Market Revenue and Forecast, by Solution (2020-2033)
18.1.5. Market Revenue and Forecast, by Technology (2020-2033)
18.1.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.1.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.1.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.1.9. Market Revenue and Forecast, by Age (2020-2033)
18.1.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.1.11. Market Revenue and Forecast, by End User (2020-2033)
18.1.12. U.S.
18.1.12.1. Market Revenue and Forecast, by Type (2020-2033)
18.1.12.2. Market Revenue and Forecast, by Application (2020-2033)
18.1.12.3. Market Revenue and Forecast, by Approach (2020-2033)
18.1.12.4. Market Revenue and Forecast, by Solution (2020-2033)
18.1.12.5. Market Revenue and Forecast, by Technology (2020-2033)
18.1.12.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.1.12.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.1.12.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.1.12.9. Market Revenue and Forecast, by Age (2020-2033)
18.1.12.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.1.12.11. Market Revenue and Forecast, by End User (2020-2033)
18.1.12.12. Market Revenue and Forecast, by December (2020-2033)
18.1.12.13. Market Revenue and Forecast, by Eleven (2020-2033)
18.1.13. Rest of North America
18.1.13.1. Market Revenue and Forecast, by Type (2020-2033)
18.1.13.2. Market Revenue and Forecast, by Application (2020-2033)
18.1.13.3. Market Revenue and Forecast, by Approach (2020-2033)
18.1.13.4. Market Revenue and Forecast, by Solution (2020-2033)
18.1.13.5. Market Revenue and Forecast, by Technology (2020-2033)
18.1.13.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.1.13.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.1.13.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.1.13.9. Market Revenue and Forecast, by Age (2020-2033)
18.1.13.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.1.13.11. Market Revenue and Forecast, by End User (2020-2033)
18.2. Europe
18.2.1. Market Revenue and Forecast, by Type (2020-2033)
18.2.2. Market Revenue and Forecast, by Application (2020-2033)
18.2.3. Market Revenue and Forecast, by Approach (2020-2033)
18.2.4. Market Revenue and Forecast, by Solution (2020-2033)
18.2.5. Market Revenue and Forecast, by Technology (2020-2033)
18.2.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.2.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.2.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.2.9. Market Revenue and Forecast, by Age (2020-2033)
18.2.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.2.11. Market Revenue and Forecast, by End User (2020-2033)
18.2.12. UK
18.2.12.1. Market Revenue and Forecast, by Type (2020-2033)
18.2.12.2. Market Revenue and Forecast, by Application (2020-2033)
18.2.12.3. Market Revenue and Forecast, by Approach (2020-2033)
18.2.12.4. Market Revenue and Forecast, by Solution (2020-2033)
18.2.12.5. Market Revenue and Forecast, by Technology (2020-2033)
18.2.12.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.2.12.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.2.12.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.2.12.9. Market Revenue and Forecast, by Age (2020-2033)
18.2.12.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.2.12.11. Market Revenue and Forecast, by End User (2020-2033)
18.2.13. Germany
18.2.13.1. Market Revenue and Forecast, by Type (2020-2033)
18.2.13.2. Market Revenue and Forecast, by Application (2020-2033)
18.2.13.3. Market Revenue and Forecast, by Approach (2020-2033)
18.2.13.4. Market Revenue and Forecast, by Solution (2020-2033)
18.2.13.5. Market Revenue and Forecast, by Technology (2020-2033)
18.2.13.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.2.13.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.2.13.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.2.13.9. Market Revenue and Forecast, by Age (2020-2033)
18.2.13.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.2.13.11. Market Revenue and Forecast, by End User (2020-2033)
18.2.14. France
18.2.14.1. Market Revenue and Forecast, by Type (2020-2033)
18.2.14.2. Market Revenue and Forecast, by Application (2020-2033)
18.2.14.3. Market Revenue and Forecast, by Approach (2020-2033)
18.2.14.4. Market Revenue and Forecast, by Solution (2020-2033)
18.2.14.5. Market Revenue and Forecast, by Technology (2020-2033)
18.2.14.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.2.14.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.2.14.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.2.14.9. Market Revenue and Forecast, by Age (2020-2033)
18.2.14.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.2.14.11. Market Revenue and Forecast, by End User (2020-2033)
18.2.15. Rest of Europe
18.2.15.1. Market Revenue and Forecast, by Type (2020-2033)
18.2.15.2. Market Revenue and Forecast, by Application (2020-2033)
18.2.15.3. Market Revenue and Forecast, by Approach (2020-2033)
18.2.15.4. Market Revenue and Forecast, by Solution (2020-2033)
18.2.15.5. Market Revenue and Forecast, by Technology (2020-2033)
18.2.15.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.2.15.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.2.15.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.2.15.9. Market Revenue and Forecast, by Age (2020-2033)
18.2.15.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.2.15.11. Market Revenue and Forecast, by End User (2020-2033)
18.3. APAC
18.3.1. Market Revenue and Forecast, by Type (2020-2033)
18.3.2. Market Revenue and Forecast, by Application (2020-2033)
18.3.3. Market Revenue and Forecast, by Approach (2020-2033)
18.3.4. Market Revenue and Forecast, by Solution (2020-2033)
18.3.5. Market Revenue and Forecast, by Technology (2020-2033)
18.3.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.3.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.3.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.3.9. Market Revenue and Forecast, by Age (2020-2033)
18.3.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.3.11. Market Revenue and Forecast, by End User (2020-2033)
18.3.12. India
18.3.12.1. Market Revenue and Forecast, by Type (2020-2033)
18.3.12.2. Market Revenue and Forecast, by Application (2020-2033)
18.3.12.3. Market Revenue and Forecast, by Approach (2020-2033)
18.3.12.4. Market Revenue and Forecast, by Solution (2020-2033)
18.3.12.5. Market Revenue and Forecast, by Technology (2020-2033)
18.3.12.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.3.12.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.3.12.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.3.12.9. Market Revenue and Forecast, by Age (2020-2033)
18.3.12.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.3.12.11. Market Revenue and Forecast, by End User (2020-2033)
18.3.13. China
18.3.13.1. Market Revenue and Forecast, by Type (2020-2033)
18.3.13.2. Market Revenue and Forecast, by Application (2020-2033)
18.3.13.3. Market Revenue and Forecast, by Approach (2020-2033)
18.3.13.4. Market Revenue and Forecast, by Solution (2020-2033)
18.3.13.5. Market Revenue and Forecast, by Technology (2020-2033)
18.3.13.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.3.13.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.3.13.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.3.13.9. Market Revenue and Forecast, by Age (2020-2033)
18.3.13.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.3.13.11. Market Revenue and Forecast, by End User (2020-2033)
18.3.14. Japan
18.3.14.1. Market Revenue and Forecast, by Type (2020-2033)
18.3.14.2. Market Revenue and Forecast, by Application (2020-2033)
18.3.14.3. Market Revenue and Forecast, by Approach (2020-2033)
18.3.14.4. Market Revenue and Forecast, by Solution (2020-2033)
18.3.14.5. Market Revenue and Forecast, by Technology (2020-2033)
18.3.14.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.3.14.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.3.14.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.3.14.9. Market Revenue and Forecast, by Age (2020-2033)
18.3.14.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.3.14.11. Market Revenue and Forecast, by End User (2020-2033)
18.3.15. Rest of APAC
18.3.15.1. Market Revenue and Forecast, by Type (2020-2033)
18.3.15.2. Market Revenue and Forecast, by Application (2020-2033)
18.3.15.3. Market Revenue and Forecast, by Approach (2020-2033)
18.3.15.4. Market Revenue and Forecast, by Solution (2020-2033)
18.3.15.5. Market Revenue and Forecast, by Technology (2020-2033)
18.3.15.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.3.15.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.3.15.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.3.15.9. Market Revenue and Forecast, by Age (2020-2033)
18.3.15.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.3.15.11. Market Revenue and Forecast, by End User (2020-2033)
18.4. MEA
18.4.1. Market Revenue and Forecast, by Type (2020-2033)
18.4.2. Market Revenue and Forecast, by Application (2020-2033)
18.4.3. Market Revenue and Forecast, by Approach (2020-2033)
18.4.4. Market Revenue and Forecast, by Solution (2020-2033)
18.4.5. Market Revenue and Forecast, by Technology (2020-2033)
18.4.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.4.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.4.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.4.9. Market Revenue and Forecast, by Age (2020-2033)
18.4.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.4.11. Market Revenue and Forecast, by End User (2020-2033)
18.4.12. GCC
18.4.12.1. Market Revenue and Forecast, by Type (2020-2033)
18.4.12.2. Market Revenue and Forecast, by Application (2020-2033)
18.4.12.3. Market Revenue and Forecast, by Approach (2020-2033)
18.4.12.4. Market Revenue and Forecast, by Solution (2020-2033)
18.4.12.5. Market Revenue and Forecast, by Technology (2020-2033)
18.4.12.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.4.12.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.4.12.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.4.12.9. Market Revenue and Forecast, by Age (2020-2033)
18.4.12.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.4.12.11. Market Revenue and Forecast, by End User (2020-2033)
18.4.13. North Africa
18.4.13.1. Market Revenue and Forecast, by Type (2020-2033)
18.4.13.2. Market Revenue and Forecast, by Application (2020-2033)
18.4.13.3. Market Revenue and Forecast, by Approach (2020-2033)
18.4.13.4. Market Revenue and Forecast, by Solution (2020-2033)
18.4.13.5. Market Revenue and Forecast, by Technology (2020-2033)
18.4.13.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.4.13.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.4.13.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.4.13.9. Market Revenue and Forecast, by Age (2020-2033)
18.4.13.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.4.13.11. Market Revenue and Forecast, by End User (2020-2033)
18.4.14. South Africa
18.4.14.1. Market Revenue and Forecast, by Type (2020-2033)
18.4.14.2. Market Revenue and Forecast, by Application (2020-2033)
18.4.14.3. Market Revenue and Forecast, by Approach (2020-2033)
18.4.14.4. Market Revenue and Forecast, by Solution (2020-2033)
18.4.14.5. Market Revenue and Forecast, by Technology (2020-2033)
18.4.14.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.4.14.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.4.14.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.4.14.9. Market Revenue and Forecast, by Age (2020-2033)
18.4.14.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.4.14.11. Market Revenue and Forecast, by End User (2020-2033)
18.4.15. Rest of MEA
18.4.15.1. Market Revenue and Forecast, by Type (2020-2033)
18.4.15.2. Market Revenue and Forecast, by Application (2020-2033)
18.4.15.3. Market Revenue and Forecast, by Approach (2020-2033)
18.4.15.4. Market Revenue and Forecast, by Solution (2020-2033)
18.4.15.5. Market Revenue and Forecast, by Technology (2020-2033)
18.4.15.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.4.15.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.4.15.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.4.15.9. Market Revenue and Forecast, by Age (2020-2033)
18.4.15.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.4.15.11. Market Revenue and Forecast, by End User (2020-2033)
18.5. Latin America
18.5.1. Market Revenue and Forecast, by Type (2020-2033)
18.5.2. Market Revenue and Forecast, by Application (2020-2033)
18.5.3. Market Revenue and Forecast, by Approach (2020-2033)
18.5.4. Market Revenue and Forecast, by Solution (2020-2033)
18.5.5. Market Revenue and Forecast, by Technology (2020-2033)
18.5.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.5.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.5.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.5.9. Market Revenue and Forecast, by Age (2020-2033)
18.5.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.5.11. Market Revenue and Forecast, by End User (2020-2033)
18.5.12. Brazil
18.5.12.1. Market Revenue and Forecast, by Type (2020-2033)
18.5.12.2. Market Revenue and Forecast, by Application (2020-2033)
18.5.12.3. Market Revenue and Forecast, by Approach (2020-2033)
18.5.12.4. Market Revenue and Forecast, by Solution (2020-2033)
18.5.12.5. Market Revenue and Forecast, by Technology (2020-2033)
18.5.12.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.5.12.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.5.12.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.5.12.9. Market Revenue and Forecast, by Age (2020-2033)
18.5.12.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.5.12.11. Market Revenue and Forecast, by End User (2020-2033)
18.5.13. Rest of LATAM
18.5.13.1. Market Revenue and Forecast, by Type (2020-2033)
18.5.13.2. Market Revenue and Forecast, by Application (2020-2033)
18.5.13.3. Market Revenue and Forecast, by Approach (2020-2033)
18.5.13.4. Market Revenue and Forecast, by Solution (2020-2033)
18.5.13.5. Market Revenue and Forecast, by Technology (2020-2033)
18.5.13.6. Market Revenue and Forecast, by Sample Type (2020-2033)
18.5.13.7. Market Revenue and Forecast, by Mode of Testing (2020-2033)
18.5.13.8. Market Revenue and Forecast, by Testing Type (2020-2033)
18.5.13.9. Market Revenue and Forecast, by Age (2020-2033)
18.5.13.10. Market Revenue and Forecast, by Distribution Channel (2020-2033)
18.5.13.11. Market Revenue and Forecast, by End User (2020-2033)
Chapter 19. Company Profiles
19.1. F-Hoffman La-Rcohe Ltd. (Switzerland)
19.1.1. Company Overview
19.1.2. Product Offerings
19.1.3. Financial Performance
19.1.4. Recent Initiatives
19.2. Danaher (US)
19.2.1. Company Overview
19.2.2. Product Offerings
19.2.3. Financial Performance
19.2.4. Recent Initiatives
19.3. BD (US)
19.3.1. Company Overview
19.3.2. Product Offerings
19.3.3. Financial Performance
19.3.4. Recent Initiatives
19.4. Thermo Fisher Scientific Inc. (US)
19.4.1. Company Overview
19.4.2. Product Offerings
19.4.3. Financial Performance
19.4.4. Recent Initiatives
19.5. ACON Laboratories Inc. (US)
19.5.1. Company Overview
19.5.2. Product Offerings
19.5.3. Financial Performance
19.5.4. Recent Initiatives
19.6. Hemosure, Inc. (US)
19.6.1. Company Overview
19.6.2. Product Offerings
19.6.3. Financial Performance
19.6.4. Recent Initiatives
19.7. MicroGen Diagnostics (US)
19.7.1. Company Overview
19.7.2. Product Offerings
19.7.3. Financial Performance
19.7.4. Recent Initiatives
19.8. Grifols, S.A (Spain)
19.8.1. Company Overview
19.8.2. Product Offerings
19.8.3. Financial Performance
19.8.4. Recent Initiatives
19.9. BODITECH MED INC. (South Korea)
19.9.1. Company Overview
19.9.2. Product Offerings
19.9.3. Financial Performance
19.9.4. Recent Initiatives
19.10. Chembio Diagnostic Systems, Inc. (US)
19.10.1. Company Overview
19.10.2. Product Offerings
19.10.3. Financial Performance
19.10.4. Recent Initiatives
Chapter 20. Research Methodology
20.1. Primary Research
20.2. Secondary Research
20.3. Assumptions
Chapter 21. Appendix
21.1. About Us
21.2. Glossary of Terms