Generic Injectable Market Garner Growth 14.1% by 2030
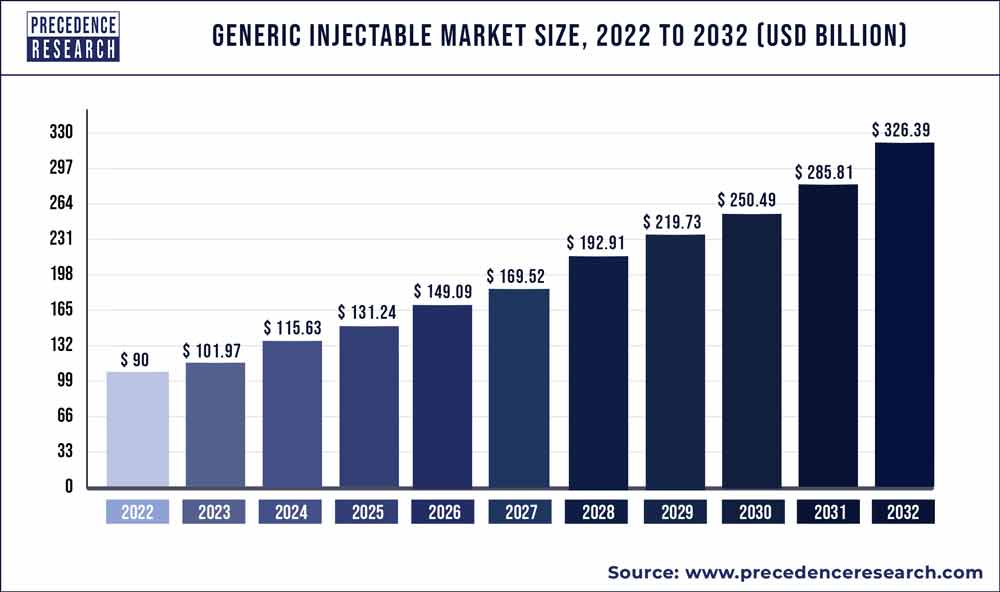
The global generic injectable market size accounted for US$ 94.3 billion in 2021 and is projected to reach around USD 309.1 billion by 2030, growing at a CAGR of 14.1% from 2022 to 2030. Driven by increased demand for generic drugs from the health care sector and the customers enhanced.
Generic Injectable Market Key Takeaway:
- The large molecule type segment has held 69% revenue share in 2021.
- The small molecule segment accounted 31% market share in 2021.
- North America region has dominated the market with revenue share of around 46% in 2021.
Report Summary
The global generic injectable market report provides a Point-by-Point and In-Depth analysis of global market size, regional and country-level market size, market share, segmentation market growth, competitive landscape, sales analysis, opportunities analysis, strategic market growth analysis, the impact of domestic and global market key players, value chain optimization, trade regulations, recent developments, product launches, area marketplace expanding, and technological innovations.
The study offers a comprehensive analysis on diverse features, including production capacities, demand, product developments, revenue generation, and sales in the generic injectable market across the globe.
A comprehensive estimate on the generic injectable market has been provided through an optimistic scenario as well as a conservative scenario, taking into account the sales of generic injectable during the forecast period. Price point comparison by region with global average price is also considered in the study.
Generic Injectable Market Scope
| Report Coverage | Details |
| Market Size in 2022 | USD 107.6 Billion |
| Market Size by 2030 | USD 309.1 Billion |
| Growth Rate from 2022 to 2030 | CAGR of 14.1% |
| Base Year | 2021 |
| Forecast Period | 2022 to 2030 |
| Segments Covered | Product Type, Molecular Type, Application, Administration, Distribution Channel, and Geography |
Injectable usually refers to a drug delivery system and acts as an alternative to oral drugs. The generic injectable is a pharmaceutical drug with the same active ingredient as the innovator drug and is equivalent to it in terms of route of administration, intended use, performance, strength, dosage, and side effects. The manufacture of generic injectables is feasible only on the expiration of the patent of innovator injectable.
The global generic injectable market is rising at a significant rate. Factors such as the growing prevalence of chronic disease, shorter & cheaper R&D cycles of the generic injectable, growing to focus on the treatment of rare disease, and increase in preference for generic injectable as compared to reference injectable are augmenting the growth of the global market.
The innovator drug is expensive owing to high expenditure on its research & development activities and thus becomes unaffordable by the consumers in developing countries. The generic injectable is extensively used in the treatment of a wide range of chronic diseases including cardiovascular diseases, osteoarthritis, respiratory diseases, cancer, diabetes, and many more while offering the same efficacy & safety as the reference injectable.
In addition to this, high margin, low investment in R&D activities, faster regulatory approvals, and with a large number of drugs expected to go off-patent, most of the manufacturers are interested to enter the generic injectable market. Thus, all these factors are majorly contributing to the growth of the global generic injectable market. Additionally, factors such as the rise in programs and initiatives by the government to spread education regarding the generic injectable, growing demand for cost-effective biologics such as monoclonal antibodies & vaccines, and increase in clinical trials are spurring the growth of the global market.
Moreover, the rise in mergers & acquisitions with the need to acquire product portfolio, pipeline development, manufacturing capabilities of existing generic injectable players to accelerate entry in the generic injectable market or consolidate market share are among the prime factors that are fostering the growth of the market. Furthermore, technological advancements in the development of the generic injectable while maintaining its safety and efficacy are likely to boost the growth of the global generic injectable market over the forecast period. However, high entry barriers characterized by complex manufacturing processes complying with stringent regulatory requirements may restrain the growth of the global generic injectable market.
The global generic injectable market is affected by the outbreak of the Covid-19 and the effect is expected to be short-term. Change in preferences is been observed in the healthcare sector during the pandemic. As the coronavirus is been rapidly infecting people across the world, services of healthcare professionals are majorly diverted for the treatment of patients infected with Covid-19. Clinical trials were temporarily halted due to strict lockdown and restriction on movement. Majorly focus was been switched towards the development of the Covid-19 vaccine. All such factors affected the production of generic injectable thereby affecting the growth of the market.
The global generic injectable market is fragmented based on molecule type, product type, route of administration, application, distribution channel, and region. Based on the molecule type, the global generic injectable market is divided into the large molecule and small molecule. Based on the product type, the global market is bifurcated into monoclonal antibodies, chemotherapy agents, peptide antibiotics, insulin, immunoglobulin, small molecule antibiotics, blood factors, cytokines, vaccines, and peptide hormones.
The route of administration segment consists of subcutaneous, intramuscular, and intravenous. Based on the application, the market is bifurcated into oncology, cardiovascular diseases, musculoskeletal disorders, diabetes, pain management, hormonal disorders, infectious diseases, CNS diseases, and blood disorders. The distribution channel segment is classified into online pharmacies, drug stores, retail pharmacies, and hospital pharmacies.
Key Highlights:
Reports Coverage: It incorporates key market sections, key makers secured, the extent of items offered in the years considered, worldwide containerized generic injectable market and study goals. Moreover, it contacts the division study gave in the report based on the sort of item and applications.
Market Outline: This area stresses the key investigations, market development rate, serious scene, market drivers, patterns, and issues notwithstanding the naturally visible pointers.
Market Production by Region: The report conveys information identified with import and fare, income, creation, and key players of every single local market contemplated are canvassed right now.
Also Read: Industrial Automation Market Size Analysis 2022 To 2030
Generic Injectable Market Players
The report includes the profiles of key generic injectable market companies along with their SWOT analysis and market strategies. In addition, the report focuses on leading industry players with information such as company profiles, components and services offered, financial information, key development in past five years.
Major companies operating in this area are:
- DR. Reddys Laboratries Ltd
- Baxter International
- Mylan N.A
- Teva Pharmaceuticals
- Astra Zeneca Plc
- Sanofi S.A
- Fresenius Kabi
- Pfizer Inc
- Cipla Ltd
- Merck & Co. Inc
- Novartis AG
- Sun Pharmaceutical Industries Ltd
- Aurobindo Pharma Limited
- Samsung Biologics Co Ltd
- Biocon
- Lupin,Ltd
- Astrazeneca
- GlaxoSmithKline Plc
- Hikma Pharmaceuticals
- Cosette Pharmaceutical, Inc
- Johnson & Johnson Services, Inc
- Sanofi SA
- Amgen Inc.
- Bristol- Myers Squibb Company
- Piramal Pharma Solutions
- Merck KGaA
Market Segmentation
By Product Type
- Chemotherapy agents
- Small molecule antibiotics
- Vaccines
- Peptide antibiotics
- Blood factors
- Peptide hormone
- Insulin
- Cytokines
- Immunoglobin
- Monoclonal Antibodies
By Molecular Type
- Small Molecule
- Large Molecule
By Application
- Oncology
- Diabetes
- Infectious Diseases
- Blood Disorders
- Musculoskeletal Disorders
- Hormonal Disorders
- Pain Management
- CNS Diseases
- Cardiovascular Diseases
By Administration
- Intravenous (IV)
- Intramuscular (IM)
- Subcutaneous (SC)
By Distribution Channel
- Hospital pharmacy
- Retail pharmacy
- Drug stores
- Online pharmacy
Regional Segmentation
- North America (U.S., Canada, Mexico)
- Europe (Germany, France, U.K., Italy, Spain, Rest of Europe)
- Asia-Pacific (China, Japan, India, Southeast Asia and Rest of APAC)
- Latin America (Brazil and Rest of Latin America)
- Middle East and Africa (GCC, North Africa, South Africa, Rest of MEA)
Research Methodology
Secondary Research
It involves company databases such as Hoover’s: This assists us to recognize financial information, the structure of the market participants and industry’s competitive landscape.
The secondary research sources referred in the process are as follows:
- Governmental bodies, and organizations creating economic policies
- National and international social welfare institutions
- Company websites, financial reports and SEC filings, broker and investor reports
- Related patent and regulatory databases
- Statistical databases and market reports
- Corporate Presentations, news, press release, and specification sheet of Manufacturers
Primary Research
Primary research includes face-to-face interviews, online surveys, and telephonic interviews.
- Means of primary research: Email interactions, telephonic discussions and Questionnaire-based research etc.
- In order to validate our research findings and analysis, we conduct primary interviews of key industry participants. Insights from primary respondents help in validating the secondary research findings. It also develops Research Team’s expertise and market understanding.
TABLE OF CONTENT
Chapter 1. Introduction
1.1. Research Objective
1.2. Scope of the Study
1.3. Definition
Chapter 2. Research Methodology
2.1. Research Approach
2.2. Data Sources
2.3. Assumptions & Limitations
Chapter 3. Executive Summary
3.1. Market Snapshot
Chapter 4. Market Variables and Scope
4.1. Introduction
4.2. Market Classification and Scope
4.3. Industry Value Chain Analysis
4.3.1. Raw Material Procurement Analysis
4.3.2. Sales and Distribution Channel Analysis
4.3.3. Downstream Buyer Analysis
Chapter 5. COVID 19 Impact on Generic Injectable Market
5.1. COVID-19 Landscape: Generic Injectable Industry Impact
5.2. COVID 19 – Impact Assessment for the Industry
5.3. COVID 19 Impact: Global Major Government Policy
5.4. Market Trends and Opportunities in the COVID-19 Landscape
Chapter 6. Market Dynamics Analysis and Trends
6.1. Market Dynamics
6.1.1. Market Drivers
6.1.2. Market Restraints
6.1.3. Market Opportunities
6.2. Porter’s Five Forces Analysis
6.2.1. Bargaining power of suppliers
6.2.2. Bargaining power of buyers
6.2.3. Threat of substitute
6.2.4. Threat of new entrants
6.2.5. Degree of competition
Chapter 7. Competitive Landscape
7.1.1. Company Market Share/Positioning Analysis
7.1.2. Key Strategies Adopted by Players
7.1.3. Vendor Landscape
7.1.3.1. List of Suppliers
7.1.3.2. List of Buyers
Chapter 8. Global Generic Injectable Market, By Product Type
8.1. Generic Injectable Market, by Product Type, 2022-2030
8.1.1. Chemotherapy agents
8.1.1.1. Market Revenue and Forecast (2017-2030)
8.1.2. Small molecule antibiotics
8.1.2.1. Market Revenue and Forecast (2017-2030)
8.1.3. Vaccines
8.1.3.1. Market Revenue and Forecast (2017-2030)
8.1.4. Peptide antibiotics
8.1.4.1. Market Revenue and Forecast (2017-2030)
8.1.5. Blood factors
8.1.5.1. Market Revenue and Forecast (2017-2030)
8.1.6. Peptide hormone
8.1.6.1. Market Revenue and Forecast (2017-2030)
8.1.7. Insulin
8.1.7.1. Market Revenue and Forecast (2017-2030)
8.1.8. Cytokines
8.1.8.1. Market Revenue and Forecast (2017-2030)
8.1.9. Immunoglobin
8.1.9.1. Market Revenue and Forecast (2017-2030)
8.1.10. Monoclonal Antibodies
8.1.10.1. Market Revenue and Forecast (2017-2030)
Chapter 9. Global Generic Injectable Market, By Molecular Type
9.1. Generic Injectable Market, by Molecular Type, 2022-2030
9.1.1. Small Molecule
9.1.1.1. Market Revenue and Forecast (2017-2030)
9.1.2. Large Molecule
9.1.2.1. Market Revenue and Forecast (2017-2030)
Chapter 10. Global Generic Injectable Market, By Application
10.1. Generic Injectable Market, by Application, 2022-2030
10.1.1. Oncology
10.1.1.1. Market Revenue and Forecast (2017-2030)
10.1.2. Diabetes
10.1.2.1. Market Revenue and Forecast (2017-2030)
10.1.3. Infectious Diseases
10.1.3.1. Market Revenue and Forecast (2017-2030)
10.1.4. Blood Disorders
10.1.4.1. Market Revenue and Forecast (2017-2030)
10.1.5. Musculoskeletal Disorders
10.1.5.1. Market Revenue and Forecast (2017-2030)
10.1.6. Hormonal Disorders
10.1.6.1. Market Revenue and Forecast (2017-2030)
10.1.7. Pain Management
10.1.7.1. Market Revenue and Forecast (2017-2030)
10.1.8. CNS Diseases
10.1.8.1. Market Revenue and Forecast (2017-2030)
10.1.9. Cardiovascular Diseases
10.1.9.1. Market Revenue and Forecast (2017-2030)
Chapter 11. Global Generic Injectable Market, By Administration
11.1. Generic Injectable Market, by Administration, 2022-2030
11.1.1. Intravenous (IV)
11.1.1.1. Market Revenue and Forecast (2017-2030)
11.1.2. Intramuscular (IM)
11.1.2.1. Market Revenue and Forecast (2017-2030)
11.1.3. Intramuscular (IM)
11.1.3.1. Market Revenue and Forecast (2017-2030)
Chapter 12. Global Generic Injectable Market, By Distribution Channel
12.1. Generic Injectable Market, by Distribution Channel, 2022-2030
12.1.1. Hospital pharmacy
12.1.1.1. Market Revenue and Forecast (2017-2030)
12.1.2. Hospital pharmacy
12.1.2.1. Market Revenue and Forecast (2017-2030)
12.1.3. Drug stores
12.1.3.1. Market Revenue and Forecast (2017-2030)
12.1.4. Online pharmacy
12.1.4.1. Market Revenue and Forecast (2017-2030)
Chapter 13. Global Generic Injectable Market, Regional Estimates and Trend Forecast
13.1. North America
13.1.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.1.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.1.3. Market Revenue and Forecast, by Application (2017-2030)
13.1.4. Market Revenue and Forecast, by Administration (2017-2030)
13.1.5. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.1.6. U.S.
13.1.6.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.1.6.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.1.6.3. Market Revenue and Forecast, by Application (2017-2030)
13.1.6.4. Market Revenue and Forecast, by Administration (2017-2030)
13.1.6.5. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.1.7. Rest of North America
13.1.7.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.1.7.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.1.7.3. Market Revenue and Forecast, by Application (2017-2030)
13.1.7.4. Market Revenue and Forecast, by Administration (2017-2030)
13.1.7.5. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.2. Europe
13.2.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.2.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.2.3. Market Revenue and Forecast, by Application (2017-2030)
13.2.4. Market Revenue and Forecast, by Administration (2017-2030)
13.2.5. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.2.6. UK
13.2.6.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.2.6.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.2.6.3. Market Revenue and Forecast, by Application (2017-2030)
13.2.7. Market Revenue and Forecast, by Administration (2017-2030)
13.2.8. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.2.9. Germany
13.2.9.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.2.9.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.2.9.3. Market Revenue and Forecast, by Application (2017-2030)
13.2.10. Market Revenue and Forecast, by Administration (2017-2030)
13.2.11. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.2.12. France
13.2.12.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.2.12.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.2.12.3. Market Revenue and Forecast, by Application (2017-2030)
13.2.12.4. Market Revenue and Forecast, by Administration (2017-2030)
13.2.13. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.2.14. Rest of Europe
13.2.14.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.2.14.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.2.14.3. Market Revenue and Forecast, by Application (2017-2030)
13.2.14.4. Market Revenue and Forecast, by Administration (2017-2030)
13.2.15. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.3. APAC
13.3.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.3.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.3.3. Market Revenue and Forecast, by Application (2017-2030)
13.3.4. Market Revenue and Forecast, by Administration (2017-2030)
13.3.5. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.3.6. India
13.3.6.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.3.6.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.3.6.3. Market Revenue and Forecast, by Application (2017-2030)
13.3.6.4. Market Revenue and Forecast, by Administration (2017-2030)
13.3.7. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.3.8. China
13.3.8.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.3.8.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.3.8.3. Market Revenue and Forecast, by Application (2017-2030)
13.3.8.4. Market Revenue and Forecast, by Administration (2017-2030)
13.3.9. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.3.10. Japan
13.3.10.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.3.10.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.3.10.3. Market Revenue and Forecast, by Application (2017-2030)
13.3.10.4. Market Revenue and Forecast, by Administration (2017-2030)
13.3.10.5. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.3.11. Rest of APAC
13.3.11.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.3.11.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.3.11.3. Market Revenue and Forecast, by Application (2017-2030)
13.3.11.4. Market Revenue and Forecast, by Administration (2017-2030)
13.3.11.5. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.4. MEA
13.4.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.4.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.4.3. Market Revenue and Forecast, by Application (2017-2030)
13.4.4. Market Revenue and Forecast, by Administration (2017-2030)
13.4.5. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.4.6. GCC
13.4.6.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.4.6.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.4.6.3. Market Revenue and Forecast, by Application (2017-2030)
13.4.6.4. Market Revenue and Forecast, by Administration (2017-2030)
13.4.7. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.4.8. North Africa
13.4.8.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.4.8.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.4.8.3. Market Revenue and Forecast, by Application (2017-2030)
13.4.8.4. Market Revenue and Forecast, by Administration (2017-2030)
13.4.9. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.4.10. South Africa
13.4.10.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.4.10.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.4.10.3. Market Revenue and Forecast, by Application (2017-2030)
13.4.10.4. Market Revenue and Forecast, by Administration (2017-2030)
13.4.10.5. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.4.11. Rest of MEA
13.4.11.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.4.11.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.4.11.3. Market Revenue and Forecast, by Application (2017-2030)
13.4.11.4. Market Revenue and Forecast, by Administration (2017-2030)
13.4.11.5. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.5. Latin America
13.5.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.5.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.5.3. Market Revenue and Forecast, by Application (2017-2030)
13.5.4. Market Revenue and Forecast, by Administration (2017-2030)
13.5.5. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.5.6. Brazil
13.5.6.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.5.6.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.5.6.3. Market Revenue and Forecast, by Application (2017-2030)
13.5.6.4. Market Revenue and Forecast, by Administration (2017-2030)
13.5.7. Market Revenue and Forecast, by Distribution Channel (2017-2030)
13.5.8. Rest of LATAM
13.5.8.1. Market Revenue and Forecast, by Product Type (2017-2030)
13.5.8.2. Market Revenue and Forecast, by Molecular Type (2017-2030)
13.5.8.3. Market Revenue and Forecast, by Application (2017-2030)
13.5.8.4. Market Revenue and Forecast, by Administration (2017-2030)
13.5.8.5. Market Revenue and Forecast, by Distribution Channel (2017-2030)
Chapter 14. Company Profiles
14.1. DR. Reddys Laboratries Ltd
14.1.1. Company Overview
14.1.2. Product Offerings
14.1.3. Financial Performance
14.1.4. Recent Initiatives
14.2. Baxter International
14.2.1. Company Overview
14.2.2. Product Offerings
14.2.3. Financial Performance
14.2.4. Recent Initiatives
14.3. Mylan N.A
14.3.1. Company Overview
14.3.2. Product Offerings
14.3.3. Financial Performance
14.3.4. Recent Initiatives
14.4. Teva Pharmaceuticals
14.4.1. Company Overview
14.4.2. Product Offerings
14.4.3. Financial Performance
14.4.4. Recent Initiatives
14.5. Teva Pharmaceuticals
14.5.1. Company Overview
14.5.2. Product Offerings
14.5.3. Financial Performance
14.5.4. Recent Initiatives
14.6. Sanofi S.A
14.6.1. Company Overview
14.6.2. Product Offerings
14.6.3. Financial Performance
14.6.4. Recent Initiatives
14.7. Fresenius Kabi
14.7.1. Company Overview
14.7.2. Product Offerings
14.7.3. Financial Performance
14.7.4. Recent Initiatives
14.8. Pfizer Inc
14.8.1. Company Overview
14.8.2. Product Offerings
14.8.3. Financial Performance
14.8.4. Recent Initiatives
14.9. Cipla Ltd
14.9.1. Company Overview
14.9.2. Product Offerings
14.9.3. Financial Performance
14.9.4. Recent Initiatives
14.10. Merck & Co. Inc
14.10.1. Company Overview
14.10.2. Product Offerings
14.10.3. Financial Performance
14.10.4. Recent Initiatives
Chapter 15. Research Methodology
15.1. Primary Research
15.2. Secondary Research
15.3. Assumptions
Chapter 16. Appendix
16.1. About Us
16.2. Glossary of Terms
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or the Asia Pacific.
Download Access to a Free Copy of Our Latest Sample Report@ https://www.precedenceresearch.com/sample/2180
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
