Generic Pharmaceuticals Contract Manufacturing Market Size, Report by 2034
The global generic pharmaceuticals contract manufacturing market size is estimated at USD 72.50 billion in 2023 and is expected to reach around USD 135.36 billion by 2034, at a CAGR of 5.84% from 2024 to 2034.
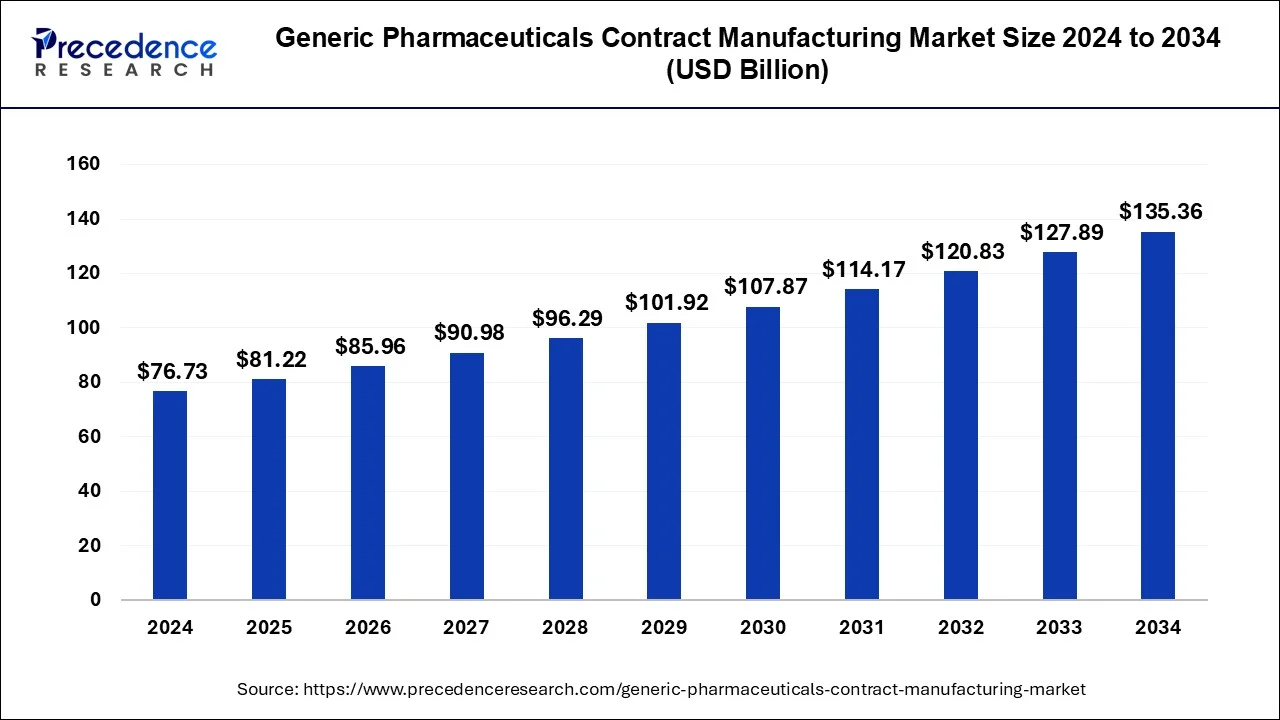
The Generic Pharmaceuticals Contract Manufacturing Market plays a pivotal role in the pharmaceutical industry by providing cost-effective solutions for producing generic medications. This sector has witnessed significant growth due to increasing demand for affordable medicines globally. Contract manufacturing organizations (CMOs) specialize in manufacturing generic drugs on behalf of pharmaceutical companies, allowing them to focus on research, development, and marketing.
Generic Pharmaceuticals Contract Manufacturing Market Key Points
- North America is estimated to be the fastest-growing during the forecast period of 2024-2033.
- By drug type, the branded generics segment has contributed more than 63% of revenue share in 2023.
- By drug type, the unbranded generics segment is significantly growing during the forecast period.
- By product, the API product segment has held a major revenue share of 58% in 2023.
- By route of administration, the oral segment has captured the largest revenue share of 62% in 2023.
- By route of administration, the parenteral segment is anticipated to be the fastest-growing during the forecast period.
- By application, the oncology segment has generated the biggest revenue share of 23% in 2023.
- By application, the immunology segment is expected to be the fastest-growing during the forecast period.
Pharmaceuticals Contract Manufacturing Market Trends
- Increasing Demand for Cost-Effective Medicines: As healthcare costs rise globally, there is a growing preference for generic drugs due to their affordability. This drives pharmaceutical companies to outsource manufacturing to contract manufacturers who can produce generics more cost-effectively.
- Advancements in Manufacturing Technologies: Contract manufacturing organizations (CMOs) are adopting advanced technologies such as continuous manufacturing and automation to improve efficiency, reduce costs, and enhance product quality. These advancements help meet stringent regulatory requirements and increase production capacity.
- Rise in Complex Drug Formulations: There is a shift towards complex generic drug formulations, including controlled-release formulations, injectables, and biosimilars. Contract manufacturers with expertise in these areas are in high demand, as pharmaceutical companies seek partners capable of handling complex processes and ensuring compliance with regulatory standards.
- Expansion of Outsourcing Activities: Pharmaceutical companies are increasingly outsourcing not only manufacturing but also research and development (R&D) to CMOs. This trend allows them to focus on core competencies such as marketing and distribution while leveraging CMOs’ expertise in manufacturing.
- Growing Regulatory Pressures: Regulatory authorities globally are becoming more stringent, requiring higher standards of quality, safety, and compliance from pharmaceutical manufacturers. Contract manufacturers must continually invest in regulatory compliance and quality assurance to meet these requirements and maintain their credibility.
Generic Pharmaceuticals Contract Manufacturing Market Scope
| Report Coverage | Details |
| Market Size by 2034 | USD 135.36 Billion |
| Market Size in 2023 | USD 72.50 Billion |
| Market Size in 2024 | USD 76.73 Billion |
| Market Growth Rate from 2024 to 2034 | CAGR of 5.84% |
| Largest Market | Asia Pacific |
| Base Year | 2024 |
| Forecast Period | 2024 to 2034 |
| Segments Covered | Drug, Product, Route of Administration, Application, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Drug Insights
The Generic Pharmaceuticals Contract Manufacturing Market encompasses a diverse range of drugs, including generic versions of branded pharmaceuticals, specialty generics, biosimilars, over-the-counter (OTC) medications, and niche pharmaceuticals. Generic drugs are produced after the patent expiration of branded drugs, offering cost-effective alternatives to consumers. Specialty generics require specialized manufacturing processes due to their complexity, while biosimilars replicate biologic drugs with advanced manufacturing capabilities. OTC drugs include non-prescription medications available in various forms, catering to consumer health needs. Niche pharmaceuticals are developed for specific markets or rare diseases, requiring tailored manufacturing approaches.
Product Types
In terms of product types, the market includes tablets, capsules, injectables, topicals, liquid orals, powders, and suppositories. Tablets and capsules are common solid oral dosage forms used for a wide range of therapeutic categories. Injectables provide sterile solutions administered intravenously, intramuscularly, or subcutaneously, crucial for delivering medications directly into the bloodstream. Topicals encompass creams, ointments, gels, and lotions applied to the skin or mucous membranes for localized effects. Liquid orals include solutions, suspensions, and syrups for oral administration, while powders are formulated for reconstitution or inhalation purposes. Suppositories are designed for rectal or vaginal administration, offering targeted delivery methods.
Route of Administration Insights
Route of administration in the Generic Pharmaceuticals Contract Manufacturing Market defines how drugs are introduced into the body. Oral administration is the most common, involving tablets, capsules, and syrups taken through the mouth for systemic absorption. Parenteral routes include intravenous, intramuscular, and subcutaneous injections, ensuring rapid drug delivery directly into the bloodstream or tissues. Topical administration involves applying medications onto the skin or mucous membranes, offering localized effects for dermatological or mucosal conditions. Inhalation routes deliver drugs into the lungs via aerosols or dry powder inhalers, beneficial for respiratory conditions. Rectal and vaginal routes utilize suppositories or creams for localized treatments in gastrointestinal or gynecological therapies.
Application Insights
In terms of application, the Generic Pharmaceuticals Contract Manufacturing Market serves various therapeutic areas such as cardiovascular, central nervous system (CNS), oncology, endocrinology, gastroenterology, respiratory, dermatology, infectious diseases, ophthalmology, urology, women’s health, pediatrics, nutraceuticals, and veterinary pharmaceuticals. Each therapeutic area requires specialized expertise in formulation development and manufacturing processes tailored to specific patient needs and regulatory requirements.
Opportunities
Opportunities in the Generic Pharmaceuticals Contract Manufacturing Market are abundant. One major opportunity lies in the growing trend towards outsourcing manufacturing to reduce costs and improve operational efficiency. Pharmaceutical companies are increasingly partnering with CMOs to leverage their expertise in manufacturing processes, regulatory compliance, and scalability. This trend is particularly pronounced in regions where labor and production costs are lower, such as Asia-Pacific and Latin America.
Additionally, the rise in patent expirations of branded drugs presents a significant opportunity for CMOs specializing in generics. As patents expire, there is a surge in the demand for affordable generic alternatives, which CMOs can capitalize on by offering flexible manufacturing solutions and quick market entry for their clients. Moreover, advancements in technology and manufacturing capabilities allow CMOs to enhance their production efficiency and maintain high standards of quality, further boosting their appeal to pharmaceutical companies seeking reliable manufacturing partners.
Challenges
However, the Generic Pharmaceuticals Contract Manufacturing Market also faces several challenges. One of the primary challenges is regulatory compliance. Pharmaceutical manufacturing is heavily regulated, and CMOs must adhere to stringent quality standards and regulatory requirements imposed by various authorities such as the FDA (Food and Drug Administration) in the United States and the EMA (European Medicines Agency) in Europe. Navigating these regulations requires significant investment in infrastructure, technology, and expertise to ensure compliance and maintain market credibility.
Another challenge is the competitive landscape. The market is highly competitive with numerous CMOs vying for contracts from pharmaceutical companies. Differentiating offerings based on quality, cost-effectiveness, and reliability becomes crucial for CMOs to secure and retain clients. Additionally, fluctuations in raw material costs, currency exchange rates, and geopolitical factors can impact operational costs and profitability for CMOs operating on a global scale.
Read Also: Facial Recognition Market Size to Reach USD 32.53 Bn by 2034
Generic Pharmaceuticals Contract Manufacturing Market Companies
- Metrics Contract Services
- Curia Global, Inc.
- Pfizer Centre One
- Syngene International Ltd.
- Acme Generics Pvt Ltd.
- Catalent, Inc.
- Alcami Corp., Inc.
- Cambrex Corp.
- Aurobindo Pharma
- Siegfried Holding AG
- Recipharm AB
- Jubilant Generics Ltd.
- Metrics Contract Services
Recent Developments
- In July 2023, Breyna Inhalation Aerosol, the first generic version of AstraZeneca’s Symbicort with an ANDA (abbreviated new drug application), was approved by the U.S. FDA (Food & Drug Administration) and launched by a global health company Viatris Inc. and Kindeva Drug Delivery L.P. for people with chronic obstructive pulmonary disease and asthma.
- In November 2023, an antibiotic manufacturing facility in Kundl, Austria was opened by a generic pharmaceuticals company based in Switzerland, Sandoz Group AG. In Europe, to strengthen the future of antibiotics manufacturing Sandoz invested $160.4m.
- In February 2024, in the United States, 5-6 new products in each quarter were planned to be launched by Alembic Pharmaceuticals, a Vadodara-based generics drugmaker.
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/
