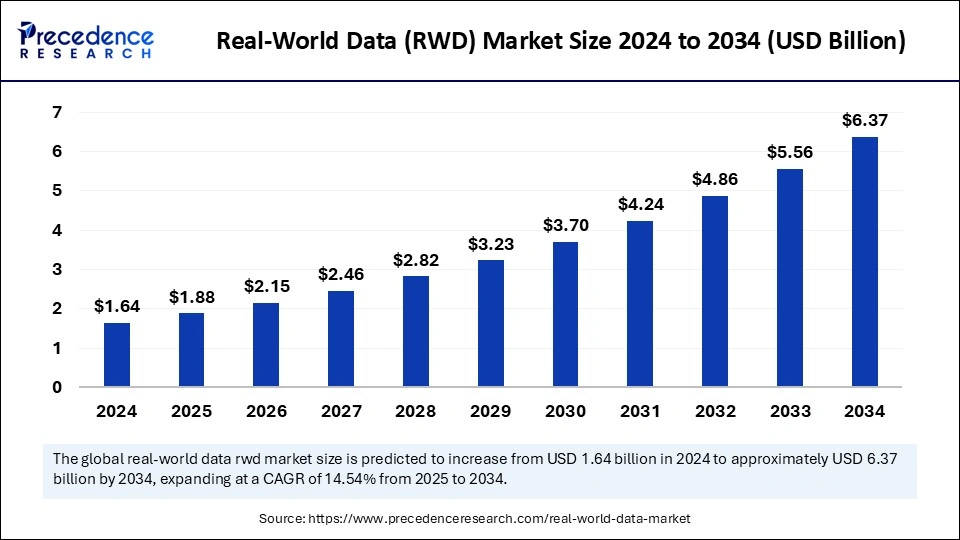
The Real-World Data (RWD) Market size is forecasted to hit USD 6.37 billion by 2034.
The global real-world data (RWD) industry is forecasted to rise from USD 1.64 billion in 2024 to around USD 6.37 billion by 2034, growing at a CAGR of 14.54%.
Real-World Data (RWD) Market Key Insights
- North America led the market by holding 43% of market share in 2024.
- Asia Pacific is projected to grow at a solid CAGR of 11% in the coming years.
- By component, the services segment held a dominant presence in the market in 2024.
- By component, the datasets segment is expected to grow at the fastest rate during the forecast period of 2024 to 2034.
- By application, the drug development and approvals segment accounted for a considerable share of the market in 2024.
- By application, the post-market surveillance segment is anticipated to grow with the highest CAGR in the market during the studied years.
- By end-user, in 2024, the pharmaceutical and medical device companies segment led the global market.
- By end-user, the healthcare providers segment is projected to expand rapidly in the coming years.
The Real-World Data (RWD) market is experiencing significant growth as industries increasingly rely on real-world evidence (RWE) to make data-driven decisions. RWD refers to data collected from real-world settings outside of traditional clinical trials, such as electronic health records (EHRs), wearable devices, insurance claims, and patient registries. The integration of RWD into healthcare, pharmaceuticals, and policy-making is transforming how treatments are assessed, improving drug development, and optimizing patient outcomes.
With advancements in artificial intelligence (AI) and big data analytics, the demand for high-quality, real-time data is expanding, leading to substantial market growth. The increasing emphasis on personalized medicine and regulatory agencies recognizing the value of RWE further fuel the market’s expansion.
Sample Link: https://www.precedenceresearch.com/sample/5694
Market Drivers
Several key factors are driving the growth of the RWD market
- Rising Demand for Real-World Evidence (RWE) – Regulatory bodies such as the FDA and EMA increasingly use RWE to support drug approvals, post-market surveillance, and regulatory decision-making.
- Advancements in Data Analytics and AI – The integration of AI and machine learning enables more accurate analysis of RWD, improving insights into treatment effectiveness and patient outcomes.
- Growing Adoption of Wearable and IoT Devices – Devices such as smartwatches and fitness trackers generate valuable health data, contributing to real-time monitoring and chronic disease management.
- Expanding Use in Pharmaceutical and Biotech Industries – Pharmaceutical companies leverage RWD to enhance drug development, identify new indications for existing drugs, and assess real-world treatment efficacy.
- Government and Policy Support – Governments and healthcare organizations are actively promoting the use of RWD for public health research, healthcare policy, and value-based care models.
Opportunities
The RWD market presents several lucrative opportunities
- Expansion in Emerging Markets – Developing countries with growing healthcare infrastructure provide new opportunities for RWD adoption, particularly in population health management.
- Personalized and Precision Medicine – RWD supports the development of personalized treatment plans by analyzing patient-specific responses to therapies.
- Integration with Blockchain for Data Security – Blockchain technology can enhance RWD integrity, security, and patient privacy, encouraging wider adoption in healthcare.
- Real-Time Decision-Making in Healthcare – The application of RWD in predictive analytics enables early disease detection and real-time treatment adjustments.
- Collaborations Between Pharma and Tech Companies – Partnerships between pharmaceutical firms and data analytics companies can accelerate innovation in drug development and patient monitoring.
Challenges
Despite its rapid growth, the RWD market faces several challenges
- Data Privacy and Security Concerns – Strict regulations such as GDPR and HIPAA require companies to ensure compliance while handling sensitive patient data.
- Data Standardization Issues – The lack of uniform data formats and integration challenges across healthcare systems hinder the seamless use of RWD.
- Quality and Reliability of Data – Inconsistent or incomplete data from diverse sources can impact the accuracy and credibility of RWE-based insights.
- Ethical and Regulatory Hurdles – Ethical concerns regarding patient consent and the use of RWD for commercial purposes require stringent oversight.
- High Costs of Data Collection and Analysis – Advanced data analytics tools and AI-driven platforms require significant investment, limiting adoption for smaller organizations.
Regional Insights
- North America – The United States dominates the RWD market due to strong regulatory support, widespread adoption of EHRs, and a well-established pharmaceutical industry. Government agencies, such as the FDA, actively use RWE for drug evaluation.
- Europe – Countries like Germany, France, and the UK are investing in digital health initiatives and data-sharing frameworks to enhance healthcare decision-making using RWD.
- Asia-Pacific – The region is witnessing rapid growth, driven by expanding healthcare infrastructure, increased adoption of digital health solutions, and government initiatives promoting data-driven healthcare policies.
- Latin America – Emerging economies such as Brazil and Mexico are leveraging RWD for improving public health programs and regulatory decision-making.
- Middle East & Africa – While the adoption of RWD is still in its early stages, the increasing focus on healthcare modernization is creating new opportunities for market expansion.
Read Also: Autoinjectors Market
