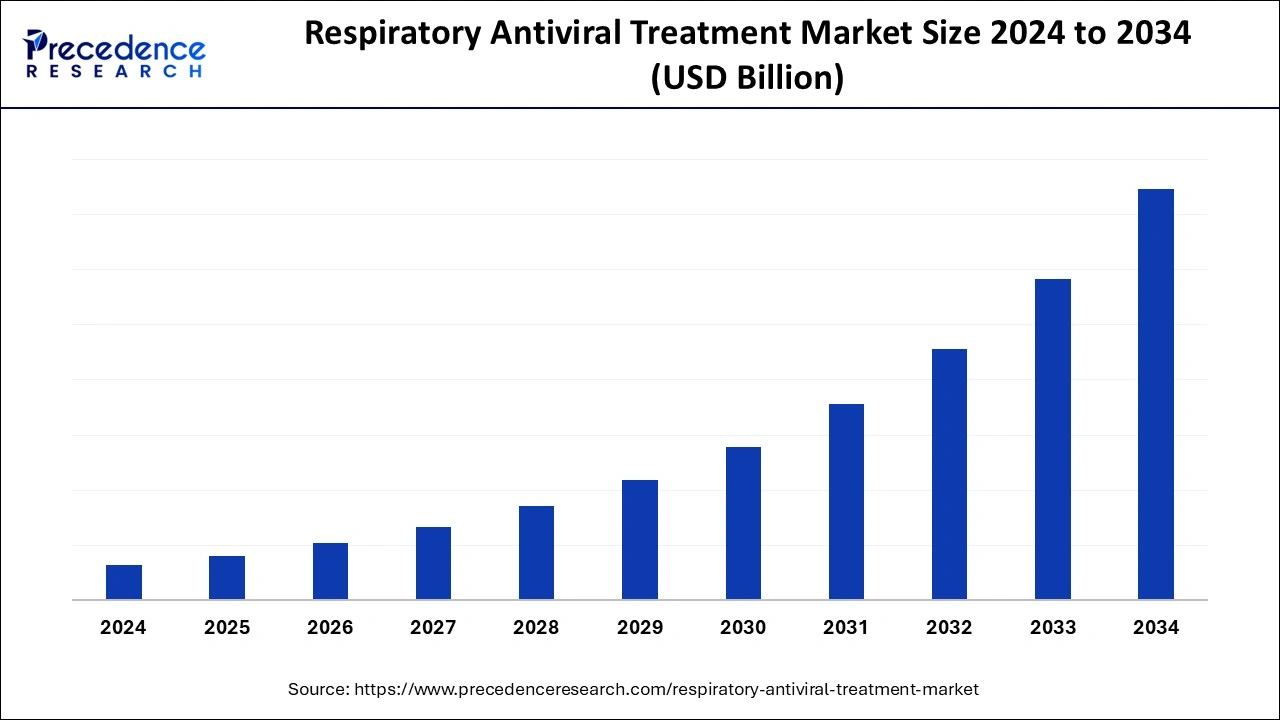
Respiratory Antiviral Treatment Market Size, Growth Report by 2033
The global respiratory antiviral treatment market is surging, with an overall revenue growth expectation of hundreds of millions of dollars from 2023 to 2032.
Key Points
- By region, North America led the respiratory antiviral treatment market with the largest share in 2023.
- By region, Asia Pacific is expected to estimate to be the fastest rate of growth during the forecast period.
- By drug class, the neuraminidase inhibitors segment led the market in 2023.
- By drug class, the nucleoside analogs segment is expected to be the fastest growing segment during the forecast period.
- By disease type, the influenza segment dominated the market in 2023.
- By distribution channel, the hospital pharmacy segment dominated the respiratory antiviral treatment market in 2023.
- By distribution channel, the retail pharmacy segment is estimated to grow with the significant CAGR during the forecast period.
The respiratory antiviral treatment market encompasses pharmaceuticals and therapies designed to combat viral infections affecting the respiratory system. These treatments are crucial in managing diseases such as influenza, respiratory syncytial virus (RSV), and COVID-19, among others. The market’s growth is driven by the increasing prevalence of respiratory viral infections globally, coupled with advancements in antiviral therapies.
Get a Sample: https://www.precedenceresearch.com/sample/4549
Growth Factors
Several factors contribute to the growth of the respiratory antiviral treatment market. Primarily, the rising incidence of viral respiratory infections worldwide has spurred demand for effective treatment options. Additionally, advancements in antiviral drug development, including novel formulations and mechanisms of action, further fuel market expansion. Moreover, the ongoing efforts to address emerging viral threats through proactive healthcare measures contribute to market growth.
Regional Insights
Regionally, North America and Europe dominate the respiratory antiviral treatment market due to high healthcare expenditures, robust research infrastructure, and early adoption of advanced therapies. Asia-Pacific is witnessing rapid market growth attributed to improving healthcare infrastructure, increasing awareness, and rising government initiatives to combat respiratory diseases.
Trends
Key trends in the respiratory antiviral treatment market include the development of broad-spectrum antiviral drugs capable of targeting multiple respiratory viruses, thereby enhancing treatment efficacy and patient compliance. Additionally, the integration of precision medicine approaches and personalized therapies are gaining traction, aiming to optimize treatment outcomes based on individual patient characteristics.
Respiratory Antiviral Treatment Market Scope
| Report Coverage | Details |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Disease Type, Drug Class, Distribution Channel, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
Respiratory Antiviral Treatment Market Dynamics
Drivers
Drivers of the respiratory antiviral treatment market include the growing elderly population susceptible to respiratory infections, escalating healthcare expenditure on infectious disease management, and expanding vaccination programs. Furthermore, the heightened focus on pandemic preparedness and rapid response strategies reinforces market growth.
Opportunities
Opportunities in the market include the development of innovative antiviral therapies with enhanced safety profiles and reduced side effects, fostering partnerships between pharmaceutical companies and research institutions to expedite drug discovery, and expanding market penetration in emerging economies with unmet healthcare needs.
Challenges
Despite growth prospects, the market faces challenges such as stringent regulatory requirements for drug approval, the emergence of drug-resistant viral strains posing therapeutic challenges, and the high cost associated with developing and commercializing antiviral treatments. Additionally, varying healthcare infrastructure and economic disparities across regions impact market accessibility.
Read Also: Nanoparticle Contract Manufacturing Market Size, Growth Report 2033
Respiratory Antiviral Treatment Market Companies
- F. Hoffmann-La Roche Ltd.
- Mylan N.V.
- Sanofi
- Pfizer Inc.
- GlaxoSmithKline plc
- Novartis AG
- Merck & Co., Inc.
- Dr. Reddy’s Laboratories Ltd.
- Zydus Cadila
- Johnson & Johnson Private Limited
- Amneal Pharmaceuticals LLC
- AbbVie Inc.
- Alembic Pharmaceuticals Limited
- Lupin
- Gilead Sciences, Inc.
- Cipla Inc.
- Bausch Health Companies Inc.
- Aurobindo Pharma
- Hetero
- Teva Pharmaceutical Industries Ltd.
Recent Developments
- In June 2024, GSK plc announced that the US Food and Drug Administration (FDA) had approved Arexvy vaccine. Arexvy vaccine is used for the prevention of RSV lower respiratory tract disease (LRTD) in adults aged between 50-59 years of age.
- In May 2024, Sanofi announced that the US Food and Drug Administration approved the extension of Dupixent (dupilumab) for three months as an add-on maintenance treatment for several adult patients suffering from uncontrolled chronic obstructive pulmonary disease (COPD).
- In February 2024, Bioxytran launched ProLectin-M. ProLectin-M is an oral antiviral treatment for treatment of COVID-19.
- In February 2024, Takeda announced that the FDA had approved EOHILIA. EOHILIA is an oral antiviral therapy for treating patients of age 11 years and above suffering from eosinophilic esophagitis (EoE).
- In May 2022, the Centre for Drug Design and Discovery (CD3) and KU Leuven’s Rega Institute for Medical Research collaborated with Gilead Sciences, Inc. This collaboration is done to develop new antiviral medications for treating patients suffering from respiratory syncytial virus (RSV) infection.
Segments Covered in the Report
By Disease Type
- Pneumonia
- Influenza
- Bronchiolitis
- Upper Respiratory Tract Infection
- Others
By Drug Class
- Nucleoside Analogs
- Neuraminidase Inhibitors
- Ion Channel Blockers
- Fusion Protein Inhibitors
By Distribution Channel
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/