RNA Therapy Clinical Trials Market Size, Growth Report by 2033
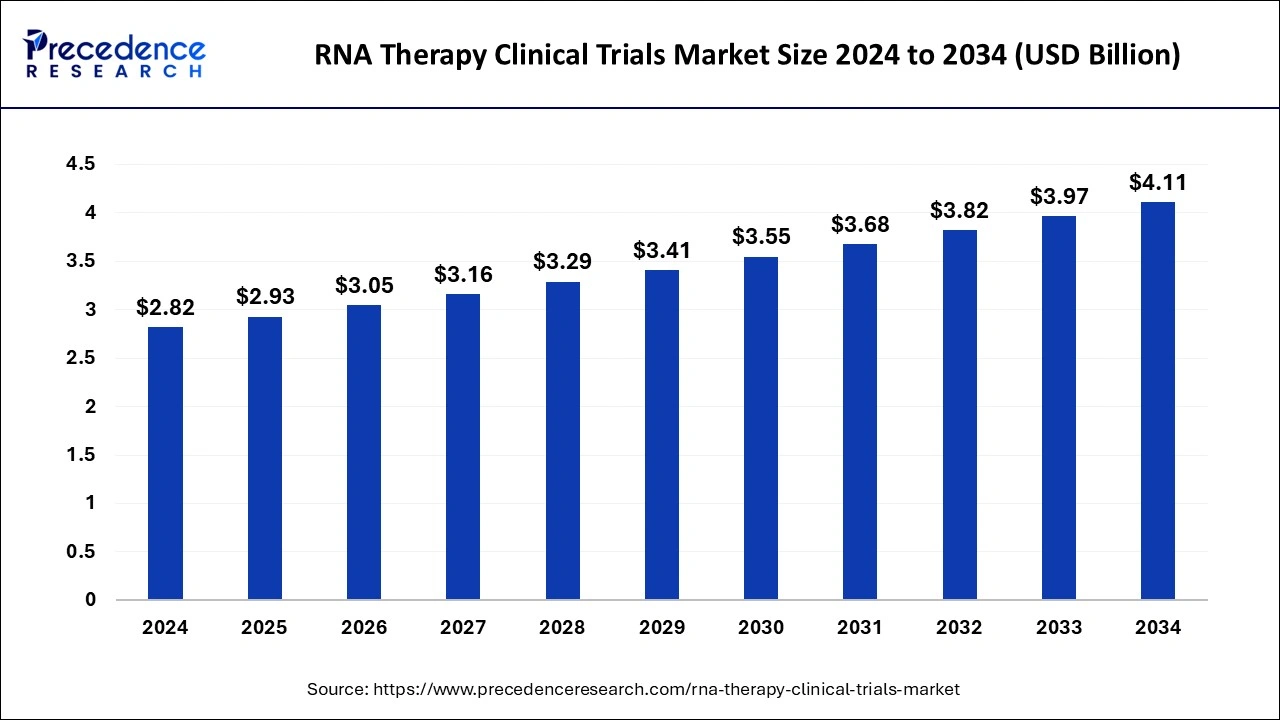
The global RNA therapy clinical trials market size is expected to increase USD 3.97 billion by 2033 from USD 2.72 billion in 2023 with a CAGR of 3.86% between 2024 and 2033.
Key Points
- The North America RNA therapy clinical trials market size reached USD 1.01 billion in 2023 and is expected to attain around USD 1.49 billion by 2033, poised to grow at a CAGR of 3.96% between 2024 and 2033.
- North America dominated the market with the largest revenue share of 37% in 2023.
- Asia Pacific is expected to expand at the fastest CAGR of 4.52% during the period studied.
- By therapeutic areas, the rare diseases segment has held a major revenue share of 22% in 2023.
- By therapeutic areas, the anticancer segment is expected to grow at the fastest rate during the forecast period.
- By modality, the messenger RNA segment has contributed more than 37% of revenue share in 2023.
- By modality, the RNA interference segment is expected to grow at a significant CAGR in the market during the forecast period.
- By phase, in 2023, the phase II segment has held a major revenue share of 43% in 2023.
- By phase the phase I segment is expected to grow substantially during the forecast period.
The RNA therapy clinical trials market is witnessing significant growth driven by advancements in RNA-based technologies, offering promising therapeutic avenues for various diseases. RNA therapies, including mRNA, siRNA, and antisense oligonucleotides, are gaining traction due to their potential to target previously undruggable targets and their role in precision medicine.
Get a Sample: https://www.precedenceresearch.com/sample/4557
Growth Factors
Key growth factors include increasing investments in biotechnology research, growing prevalence of chronic diseases such as cancer and genetic disorders, and advancements in drug delivery systems enhancing RNA therapy efficacy and safety profiles.
RNA Therapy Clinical Trials Market Trends
- Increasing Research and Development: There has been a significant rise in research and development activities focused on miRNA-based therapies. This includes both preclinical studies and clinical trials across various therapeutic areas.
- Diverse Therapeutic Applications: MiRNA therapies are being explored for a wide range of therapeutic applications, including cancer, cardiovascular diseases, neurological disorders, and metabolic diseases. The versatility of miRNAs in regulating gene expression makes them attractive candidates for innovative treatments.
- Advanced Delivery Systems: Advancements in delivery systems are crucial for the success of miRNA therapies. Researchers are focusing on developing efficient delivery methods that ensure targeted delivery to specific tissues and cells while maintaining therapeutic efficacy.
- Collaborations and Partnerships: Collaboration between pharmaceutical companies, biotech firms, academic institutions, and research organizations is increasing. These partnerships aim to leverage expertise and resources to accelerate the development of miRNA therapies from bench to bedside.
- Regulatory Developments: Regulatory agencies are actively engaged in providing guidelines and frameworks for the development and approval of miRNA-based therapeutics. This regulatory support is essential for advancing these therapies through clinical trials and eventual commercialization.
- Patient-Centric Approaches: There is a growing emphasis on patient-centric approaches in clinical trials, including patient recruitment strategies, personalized medicine aspects, and patient-reported outcomes. These approaches are crucial for optimizing treatment efficacy and patient satisfaction.
Region Insights:
North America and Europe dominate the RNA therapy clinical trials market due to robust healthcare infrastructure, substantial R&D investments, and supportive regulatory frameworks fostering clinical trials. Emerging economies in Asia-Pacific are also witnessing rapid growth, driven by expanding biopharmaceutical industries and rising healthcare expenditures.
RNA Therapy Clinical Trials Market Scope
| Report Coverage | Details |
| Market Size by 2033 | USD 3.97 Billion |
| Market Size in 2023 | USD 2.72 Billion |
| Market Size in 2024 | USD 2.82 Billion |
| Market Growth Rate from 2024 to 2033 | CAGR of 3.86% |
| Largest Market | North America |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Modality, Clinical Trials Phase, Therapeutic Areas, and Regions |
| Regions Covered | North America, Europe, Asia-Pacific, Latin America, and Middle East & Africa |
RNA Therapy Clinical Trials Market Dynamics
Drivers:
Major drivers include the demand for personalized medicine, advancements in RNA sequencing technologies, and collaborative efforts among pharmaceutical companies and research institutions to develop innovative RNA-based therapies.
Opportunities:
Opportunities abound in expanding applications of RNA therapies beyond oncology to include rare genetic diseases, infectious diseases, and neurological disorders. Moreover, the potential for RNA vaccines and gene editing technologies presents novel avenues for market expansion.
Challenges:
Challenges include technological complexities in RNA synthesis and delivery, potential off-target effects, regulatory challenges associated with RNA-based therapies, and high costs of clinical trials and therapy development.
Read Also: Microwave Devices Market Size to Worth USD 14.13 Bn by 2033
RNA Therapy Clinical Trials Market Companies
- IQVIA
- ICON Plc.
- Laboratory Corporation of America Holdings
- Charles River Laboratories International, Inc.
- PAREXEL International Corp.
- Syneos Health
- Medpace Holdings, Inc.
- PPD Inc.
- Novotech
- Veristat, LLC.
Recent Developments
- In June 2023, Charles River Laboratories International, Inc. and Curigin established an alliance to manufacture adenoviral vectors. The gene therapy company will rely on Charles River’s market-leading experience in contract development and manufacturing organization (CDMO) solutions to support its preclinical and clinical studies.
- In June 2023, Moderna got the FDA’s green light for its mRNA-1273 vaccine. At the same time, Pfizer was also granted approval for their BNT162b2 vaccine. These vaccines were designed against COVID-19 for children aged from six months to five years old.
- In March 2023, Moderna submitted an IND application to the FDA regarding their mRNA-1273 vaccine against respiratory syncytial virus (RSV). This vaccine has helped reduce the possibility of respiratory tract infections in young children and infants.
Segments Covered in the Report
By Modality
- RNA Interference
- Antisense Therapy
- Messenger RNA
- Oligonucleotide, Non-antisense, Non-RNAi
By Clinical Trials Phase
- Phase I
- Phase II
- Phase III
- Phase IV
By Therapeutic Areas
- Rare Diseases
- Anti-infective
- Anticancer
- Neurological
- Alimentary/Metabolic
- Musculoskeletal
- Cardiovascular Respiratory
- Sensory
- Others
By Geography
- North America
- Asia Pacific
- Europe
- Latin America
- Middle East & Africa
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/