U.S. Point of Care Infectious Disease Testing Market Size, Share, Report 2033
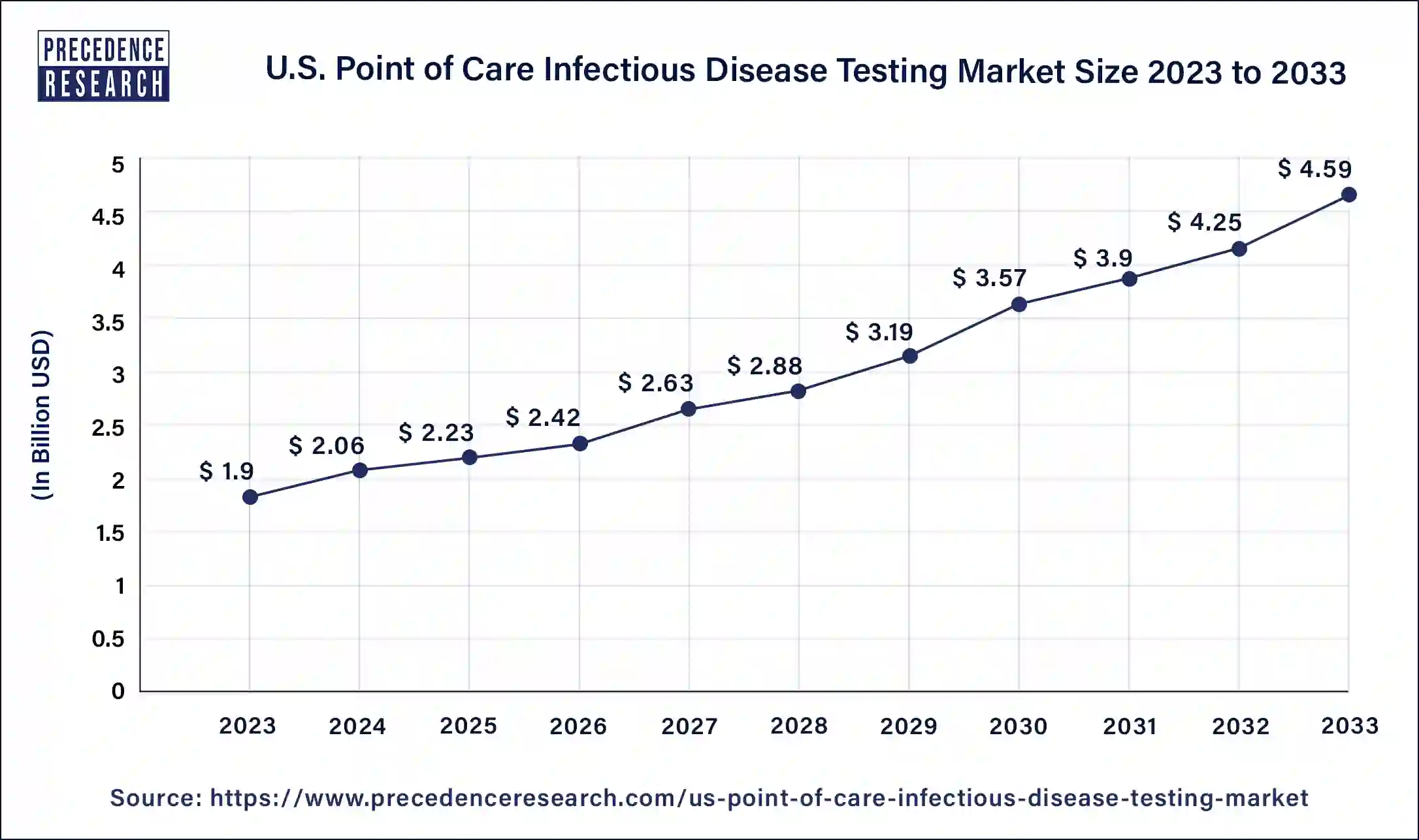
The U.S. point of care infectious disease testing market size is expected to increase USD 4.59 billion by 2033 from USD 1.9 billion in 2023 with a CAGR of 9.30% between 2024 and 2033.
Key Points
- By disease, the influenza/flu segment has held a major revenue share of 24.42% in 2023.
- By disease, the respiratory syncytial virus (RSV) segment is the second largest in the market in 2023.
- By end-user, the hospitals segment dominated market with the largest revenue share of 38.56% in 2023.
The U.S. Point of Care (POC) infectious disease testing market has witnessed significant growth in recent years, driven by advancements in diagnostic technologies, increasing prevalence of infectious diseases, and the need for rapid and accurate diagnostic solutions. Point of care testing refers to medical diagnostic testing at or near the site of patient care, providing immediate results that enable prompt clinical decision-making. In the context of infectious diseases, POC testing plays a crucial role in early detection, monitoring, and management, thereby improving patient outcomes and reducing transmission risks.
Get a Sample: https://www.precedenceresearch.com/sample/4461
Growth Factors
Several key factors contribute to the growth of the U.S. POC infectious disease testing market. Firstly, technological innovations have led to the development of highly sensitive and specific diagnostic tools capable of detecting a wide range of pathogens rapidly. These advancements include molecular diagnostics, immunochromatography, and nucleic acid amplification techniques, which have significantly enhanced the accuracy and speed of POC testing. Moreover, the growing demand for decentralized healthcare solutions, coupled with increasing patient preference for quick and convenient diagnostic options, has bolstered the adoption of POC infectious disease testing across various healthcare settings.
Government initiatives and supportive regulatory frameworks have also played a pivotal role in market expansion. Programs aimed at controlling infectious disease outbreaks, such as influenza and COVID-19, have underscored the importance of POC testing in epidemic preparedness and response. Furthermore, collaborations between healthcare providers, diagnostic companies, and research institutions have facilitated the development of innovative POC testing platforms, driving market growth through enhanced product offerings and expanded market reach.
Region Insights
In the U.S., the demand for POC infectious disease testing varies across different regions based on demographic trends, healthcare infrastructure, and disease prevalence. Urban centers with high population density often exhibit greater adoption rates of POC testing due to accessibility and the need for rapid disease management. Rural areas, on the other hand, may face challenges related to healthcare access and infrastructure limitations, impacting the penetration of POC testing technologies. Regional disparities in healthcare expenditure and reimbursement policies also influence market dynamics, with certain states or regions demonstrating higher healthcare spending and easier access to POC testing services.
U.S. Point of Care Infectious Disease Testing Market Scope
| Report Coverage | Details |
| Market Size in 2023 | USD 1.9 Billion |
| Market Size in 2024 | USD 2.06 Billion |
| Market Size by 2033 | USD 4.59 Billion |
| Market Growth Rate from 2024 to 2033 | CAGR of 9.30% |
| Base Year | 2023 |
| Forecast Period | 2024 to 2033 |
| Segments Covered | Disease and End-user |
U.S. Point of Care Infectious Disease Testing Market Dynamics
Drivers
Several drivers propel the growth of the U.S. POC infectious disease testing market. Firstly, the increasing incidence of infectious diseases such as influenza, sexually transmitted infections (STIs), and emerging infectious threats like Zika virus and Ebola highlight the critical need for rapid and accurate diagnostic solutions. POC testing enables timely diagnosis and treatment initiation, thereby reducing disease transmission rates and improving public health outcomes. Additionally, the shift towards personalized medicine and patient-centered care models emphasizes the role of POC testing in delivering targeted therapies and optimizing treatment outcomes.
Advancements in healthcare infrastructure and technology also drive market expansion. Integration of electronic health records (EHRs), telemedicine platforms, and mobile health applications facilitates seamless data management and remote patient monitoring, enhancing the utility and scalability of POC testing solutions. Moreover, increasing investments in research and development (R&D) by pharmaceutical companies and diagnostic manufacturers stimulate product innovation and the introduction of next-generation POC testing platforms, further propelling market growth.
Opportunities
The U.S. POC infectious disease testing market presents several opportunities for stakeholders across the healthcare spectrum. Expansion of testing capabilities beyond traditional infectious diseases to include emerging pathogens and antimicrobial resistance profiling represents a promising avenue for market growth. Furthermore, the integration of artificial intelligence (AI) and machine learning algorithms into POC testing devices holds potential for enhancing diagnostic accuracy and predictive analytics, thereby optimizing clinical decision-making and patient management.
Strategic collaborations and partnerships between diagnostic companies and healthcare providers can facilitate the development of customized POC testing solutions tailored to specific disease profiles and patient populations. Moreover, the increasing focus on point-of-care diagnostics in non-traditional settings such as pharmacies, urgent care centers, and community clinics opens up new market opportunities by expanding access to POC testing services and improving healthcare delivery efficiency.
Challenges
Despite its growth prospects, the U.S. POC infectious disease testing market faces several challenges. Regulatory complexities and stringent approval processes for new diagnostic technologies can delay market entry and limit product innovation. Variability in test performance and accuracy across different POC platforms also pose challenges in standardizing diagnostic protocols and ensuring consistent quality control measures. Moreover, economic constraints and healthcare budget constraints may restrict the widespread adoption of POC testing technologies, particularly in underserved or economically disadvantaged populations.
Addressing these challenges requires concerted efforts from regulatory bodies, healthcare providers, and industry stakeholders to streamline regulatory pathways, enhance quality assurance measures, and promote equitable access to POC testing services. Furthermore, ongoing investments in research and development, coupled with education and training programs for healthcare professionals, are essential to advancing the capabilities and reliability of POC infectious disease testing technologies.
Read Also: Credit Card Payments Market Size to Worth USD 1,331.50 Bn By 2033
U.S. Point of Care Infectious Disease Testing Market Companies
- Beckton Dickinson
- Roche
- Biomerieux
- Siemens
- Bio-rad
- Chembio Diagnostic Systems
- Quidel Corporation
- Abbott
Recent Developments
- In March 2024, in spring 2024, the City University of New York (CUNY) Institute for Implementation Science in Population Health (ISPH) and the CUNY Graduate School of Public Health and Health Policy (CUNY SPH) will launch a critical two-year prospective epidemiologic study in partnership with Pfizer to monitor acute respiratory infections nationwide.
- In February 2022, The World Health Organization (WHO) approved Trinity Biotech plc’s new HIV screening tool, Trin Screen HIV.
Segments Covered in the Report
By Disease
- Pneumonia Or Streptococcus Associated Infections
- Respiratory Syncytial Virus (RSV)
- TB and Drug Resistant TB POC
- Influenza/Flu POC
- HIV POC
- Others
By End-user
- Hospitals
- Clinics
- Home
- Assisted Living Healthcare Facilities
- Laboratories
- Others
Contact Us:
Mr. Alex
Sales Manager
Call: +1 9197 992 333
Email: sales@precedenceresearch.com
Web: https://www.precedenceresearch.com
Blog: https://www.expresswebwire.com/
Blog: https://www.uswebwire.com/